AHA, BHA & PHA
EXFOLIATION – PEELING
Exfoliation is a process by which the top layer of cells in the skin's surface is rejected, which is a natural process for the skin. This process is also called "desquamation" and is connected with the ongoing turnover of the upper skin layer (epidermal turnover), which is important for both the skin's appearance and properties. With age, the speed of these processes decreases, which contributes to changes in the skin. Exfoliation or peeling of the skin can remedy certain changes and troubles in the skin. There are generally two methods for exfoliating the skin from the outside: Chemical and mechanical. Mechanical exfoliation can, for example, be carried out using a scrub, which physically rubs the top skin cells loose, while chemical exfoliation is carried out using special substances that chemically penetrate and loosen the top skin cells.
PUCA - PURE & CARE has a range of products for exfoliating the skin. Read more here.
Products with AHA, BHA, PHA
WHAT IS AHA, BHA & PHA?
AHA, BHA and PHA are groups of chemically exfoliating substances, which have been shown in many studies to be able to remedy, for example, wrinkles, uneven skin tone and various skin problems. In a cosmetic context, the goal is often to achieve an anti-aging effect, where in dermatology it is often to remedy various skin diseases such as ichthyosis, acne, psoriasis and warts; but can also be of a more cosmetic nature, such as treatment of sun-damaged skin and scars. Many studies with these substances have been carried out in dermatological contexts, where the concentrations of the chemical agents are very high compared to what is used in cosmetics and where the treatment is carried out by professionals.
AHA, BHA and PHA are abbreviations for Alpha Hydroxy Acid, Beta Hydroxy Acid and Poly Hydroxy Acid respectively - these are three groups of substances which are collectively called Hydroxy Acid (HA) - Hydroxy Acids. What they have in common is that they contain a carboxylic acid group and one or more hydroxy groups in the chemical structure. Within the group of HAs, you can also come across the abbreviation "AMA", which stands for Aromatic Hydroxy Acids, which contains a so-called aromatic ring, "LHA" which stands for Lipo Hydroxy Acid, which is a group of HAs where the molecule is made more fat-soluble by e.g. adding a fatty acid to an HA structure and the designations "bionic acid" (sometimes abbreviated PHBA) or "aldonic acids", which are a subgroup of PHAs where a sugar molecule is attached to the PHA-structure. Here the focus will be on AHA, BHA and PHA, of which it can generally be said that AHA often works more powerfully than BHA, which is slightly stronger than PHA - but the effects depend on exactly which substance is used, the concentration of the substance, the overall product (the formulation), the pH value of the product and the nature of the skin. Several of the substances in each group, in addition to being exfoliating, are also moisturizers, metal binders and antioxidants; and some of them are found in nature and in the body. In several of its products, PUCA PURE & CARE uses the following substances from the groups of AHA, BHA and PHA: Glycolic Acid, Lactic Acid (1*), Salicylic Acid (2*), Tartaric Acid (3*) and Gluconolactone and also Citric Acid (4*) for pH adjustment of products.
EPIDERMAL TURNOVER AND CHEMICAL EXFOLIATION OF THE SKIN
The epidermis is the outermost layer of the skin - below that is the dermis and below that the subcutis or hypodermis. The epidermis consists of 5 layers: At the bottom is the stratum basale, which is a single cell layer of i.a. melanocytes and stem cells that constantly form new keratinocytes (cells). These keratinocytes migrate outwards and gradually form the other epidermal layers, which are:
(1*) Lactic acid is produced by microbial fermentation followed by purification.
(2*) Salicylic acid is produced by chemical synthesis followed by purification.
(3*) Tartaric Acid is produced by chemical synthesis followed by purification.
(4*) Citric Acid is produced by microbial fermentation followed by purification.
Stratum spinosum, stratum granulosum, stratum lucidum and finally the outermost layer is the stratum corneum (SC), which is approx. 10-30 µm thick and consists of 15-20 layers of flat dead skin cells called corneocytes, which are gradually rejected (exfoliated). Thus, the skin's epidermis is renewed all the time: the epidermal turnover – i.e. from the keratinocyte being formed in the stratum basale until it is rejected from the stratum corneum as a corneocyte – takes about twice as many days in aged skin compared to young skin. With age, the stratum corneum normally becomes thicker, but the other layers in the epidermis become thinner so that the epidermis generally becomes thinner.
During the migration, the keratinocytes produce a substantial amount of Keratin (a group of protein molecules), which will make up a large part of the corneocytes in the stratum corneum. This keratinization and desquamation process is important for the structural integrity of the cells and the skin barrier; thus, changes in these can lead to different types of skin problems. Hyperkeratinization takes place when the desquamation process does not go fast enough so that too many corneocytes accumulate on the surface and in pores, which is a contributing cause of e.g. keratosis, ichthyosis and acne. It may be related to dry skin, as the effect of the keratolytic enzymes, which participate in the desquamation process, is dependent on a moist environment. In addition to corneocytes, the stratum corneum also contains various fats and special structures that bind the cells together. Changes in the fats or these connective structures can also affect the skin's barrier properties and result in skin problems.
HELP THE SKIN´S OWN EXFOLIATION ON THE WAY
Chemical exfoliation can assist the skin’s own exfoliation process and improve its appearance, removing old skin cells from the surface so that the newer layer of skin underneath can shine through. The principle is a controlled chemical removal of the outermost skin cells. Substances that can accomplish this are said to have keratolytic properties. In cosmetic contexts, it is usually only the outermost skin cells that are loosened, whereas in dermatological contexts deeper damage may be caused to the skin in order to help regenerate it. Dermatology uses superficial, medium and deep peeling or exfoliation, where the strongest agents can reach down to the dermis and actually cause visible damage to the skin, which can be associated with a certain risk of side effects and may require special aftercare. Treatments like this are performed by professionals under controlled conditions.
AHA, BHA & PHA?
– THE CHEMICAL STRUCTURE
AHAs, BHAs and PHAs are defined by their chemical structure, which contains a carboxylic acid group and one or more alcohol groups (hydroxy groups). A carboxylic acid group consists of a Carbon atom with a double bond to an Oxygen atom and a single bond to a Hydroxy group (OH group). A carboxylic acid group is often written like this: -COOH. See Figure 1.
For AHAs, an alcohol group is placed on the Carbon atom next to the carboxylic acid group - this Carbon atom is called the alpha position (α) - hence the name Alpha Hydroxy Acid, AHA. Carboxylic acid is normally a relatively weak acid, but the OH group in the alpha position can form an internal hydrogen bond with one of the oxygen atoms in the carboxylic acid, which has the effect of making the acid a notch stronger.
For BHAs, the alcohol group is located on the next Carbon atom – the beta position (β) – this can also form an internal hydrogen bond with one of the Oxygen atoms in the carboxylic acid, making the acid a little stronger. If a molecule has an alcohol group in both the alpha and beta positions (and no more) it is normally classified as an AHA, although it can also be said to be a BHA. Finally, there are the PHAs, which contain a minimum of two (often more) alcohol groups and usually one of them is in the alpha position.
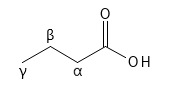
Figure 1: Chemical structure of a simple carboxylic acid, indicating the positions alpha (α), beta (β) and gamma (γ).
Chemically, the carboxylic acid group in AHA, BHA and PHA depends on the pH of the environment. Thus, at relatively low pH values it will be in acid form (-COOH) and at higher pH values will be in the form of the corresponding base form, where the Hydrogen atom is split off (-COO-). The pH value at which the Hydrogen atom is split off depends on the properties of the molecule and this gives a liquid transition so that over a certain pH range there will be a balance between the acid form and the corresponding base form. In general, a strong acid gives off the Hydrogen atom at a lower pH than a weaker acid – thus a fairly low pH value is needed for a strong acid to remain in the acid form. In this way, the pH value of the product determines which form the acid is in: The lower the pH, the more of the acid is in acid form, which is not negatively charged; the corresponding base is negatively charged. This can have an impact on how powerful the substance works and how well it penetrates the skin, as a negative charge can inhibit skin absorption (5*). Thus, a low pH value can also increase the risk of irritation and the burning sensation that can occur, for example, with AHA exfoliation with a low pH. There are also other factors that affect how easily a substance gets into the skin – for example the size of the molecule and the overall formulation of the product used.
In the following sections, AHA, BHA and PHA and representatives from each group will be presented.
(5*) An interesting topic in relation to the skin and pH is that it has been shown that there is a pH gradient inside the epidermis: On the outside (the surface of the stratum corneum) the pH is typically 4.5-5.7, while the pH in the living part of the epidermis (below the stratum corneum) is about 7. This means that if an acid enters the stratum corneum, it will dissociate into its corresponding base form.
AHA – ALPHA HYDROXY ACIDS
AHA has been known and used for many years – both in dermatology and in cosmetics. The purpose of using AHA is primarily to exfoliate; moreover, several of them are moisturizing and some of them are also used in low concentrations as metal binders and for pH regulation of the finished product. The most known and used AHAs are:
- Citric Acid
- Glycolic Acid
- Lactic Acid
- Malic Acid
- Mandelic Acid
- Tartaric Acid
GET RESILIENT SKIN WITH AHA
They occur naturally, but in many cases the substances used are produced by chemical synthesis or by the use of microorganisms.
Their effects (and side effects) depend on which AHA(s) are used, the concentration, the pH of the product, the composition of the whole product and the nature of the skin. Studies have shown that AHAs can have an anti-ageing effect in the form of smoother skin tone, fewer wrinkles and more elastic skin and reduce skin problems such as acne. The mechanisms behind the effects are not fully understood, but there are believed to be several mechanisms at play, which probably depend on which molecule is used, the concentration, pH etc. One of the mechanisms behind this is probably their hygroscopic property: that they can bind water and thus increase the water capacity of the skin. As mentioned, water is important for the keratolytic enzymes that contribute to the desquamation process. Another way in which AHAs are believed to affect the desquamation process is more directly: by interacting with intercellular ionic bonds and thus reducing cohesion between the corneocytes and thus acting keratolytically. The metal-binding effect of the AHAs is also thought to play a role in their exfoliating effect: by binding calcium ions, fewer free calcium ions are available for the calcium-dependent protein structures that otherwise bind the cells together, so the cells are loosened. The actual reduction of pH on the surface, which an acidic product with a relatively low pH can provide for some time, is also believed to contribute to the promotion of desquamation. Studies also suggest that AHAs can work by affecting deeper layers of the epidermis and dermis. In vitro studies using human skin fibroblasts have shown that, depending on the dose, AHAs can increase cell proliferation and improve differentiation and thus promote epidermal turnover and cell renewal, which should promote an even skin tone. In addition, some studies suggest that they can increase the skin's production of ceramides, collagen and glycosaminoglycans (e.g. hyaluronic acid), which can alleviate wrinkles.
Despite the fact that the top cell layer is exfoliated, the overall conclusion from various studies is that cosmetic products with AHA (not high concentration or very low pH) do not noticeably compromise the skin barrier.
In general, cosmetic AHA products are safe to use. The side effects that can occur – especially with high concentration and low pH value – are a stinging or burning sensation, rash, swelling, pigment changes (especially with highly pigmented skin), blisters, itching and irritation. And then there are discussions about whether AHAs can increase the skin's sensitivity to the sun and thus increase sun-induced damage. Some studies (especially older studies) have shown that exfoliation can increase the skin's sensitivity (a reversible effect), e.g. in the form of increased redness after UV radiation, while others have shown that e.g. Citric Acid, Glycolic Acid and Malic acid can have a sun-protective effect e.g. by reducing certain proinflammatory cytokines. Due to conflicting results from different studies, this is still not clear and therefore there are often warnings or recommendations to protect yourself from sunlight after using products with AHA.
Both expert panels from the USA and the EU have given assessments of AHAs and guidelines for use in cosmetics. In 2017, the American experts concluded that their assessment from 1998 was still valid, concluding that Glycolic Acid and Lactic Acids (as well as their salts and simple esters) are safe to use in cosmetic products up to 10% with a minimum pH of 3.5 - if they are formulated in a way to avoid increased sun sensitivity or if the instructions for use include daily sun protection. In relation to use by professionals, the Americans conclude that up to 30% AHA and a minimum pH of 3.0 are safe to use if the product is for short-term use after thorough washing and sun protection is used afterwards. The assessment by the EU's expert panel in 2004 maintains their assessment from 2000, which follows stricter precautionary principles compared to the Americans'. Their assessment is that you can safely use Glycolic Acid up to 4% at a minimum pH of 3.8 and for Lactic Acid it is a maximum of 2.5% at a minimum pH of 5.0. In addition, it is recommended that there should be a warning not to get it in the eyes and to avoid or protect oneself from UV radiation. In both the USA and the EU, people have primarily looked at Glycolic Acid and Lactic Acid, as they are the most used AHAs and are believed to be the most powerful. In the following, representatives from the group of AHAs will be described.
PUCA - PURE & CARE has a range of products containing AHA. Find dem all here.
CITRIC ACID
Citric Acid is found, for example, in citrus fruits and was first isolated in 1784 from lemon juice. It is also found naturally in the body and in most other organisms on earth. Today, it is primarily produced via microbial fermentation – often using specific strains of the mold Aspergillus niger. Stoffet anvendes fx meget i kosmetik – primært til at regulere pH og binde metaller, hvilket hjælper konserveringen – og i fødevarer med samme formål og for at give smag. Som fødevaretilsætningsstof går det under betegnelsen E330. Det er et farveløst til hvidt fast, vandopløseligt stof. I forhold til de andre AHA’er er Citric Acid et lidt større molekyle med tre carboxylsyre-grupper og en OH-gruppe i midten af molekylet, som er i alpha-position i forhold til den midterst carboxylsyre-gruppe og i beta-position i forhold til de to andre carboxylsyre-grupper – se figur 2. Man har vist, at Citric Acid har keratolytiske egenskaber, men det bruges ikke så tit til eksfoliering af huden og i forhold til de andre AHA’er er der ikke lavet mange studier i den henseende med Citric Acid.
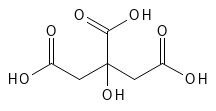
Figure 2: The chemical structure of Citric Acid.
GLYCOLIC ACID
Glycolic Acid is the most used of the AHAs for exfoliation and it is often this substance that studies have been made with. It is found, for example, in sugar cane, grapes and sugar beet and can be isolated from such natural sources or produced by chemical synthesis or via enzymatic biochemical process. The first time it was produced was in 1851. As can be seen from Figure 3, it is the smallest possible AHA and the small molecular weight is one of the contributing factors to its effect, as small molecules generally penetrate the skin more easily. In its pure form, it is a colorless to white, odorless and water-soluble solid. It is hygroscopic and also a metal binder (e.g. binds copper particularly well) and in addition to cosmetic use, it is also used in the textile industry in dyeing processes.
Cosmetically, Glycolic Acid , is an interesting substance with its properties and the effects studies have shown – some of them, however, at rather high concentrations compared to cosmetics. It has been shown, for example, that Glycolic Acid can smooth the skin (reduce wrinkles), promote an even skin tone (reduce pigment spots), increase skin firmness and elasticity, and reduce acne and scars. Glycolic Acid has been shown to have a protective effect against the harmful effects of the sun (photo-protective) at relatively low concentrations. Conversely, some studies have shown that Glycolic Acid at high concentrations can have a negative effect in relation to the influence of the sun (photo-toxic).
The mechanisms have also been investigated in many studies. In Vitro studies indicate that Glycolic Acid has an anti-inflammatory effect and can downregulate UVB-induced cytokine and chemokine secretion from keratinocytes, which are contributing factors in sun effects.
Glycolic Acid has a clear keratolytic (exfoliating) effect by, for example, promoting the breakdown of the structures (corneodesmosomes) that bind the corneocytes together. It has also been shown that Glycolic Acid can increase the level of both dermal and epidermal hyaluronic acid, stimulate collagen production, improve the quality of the elastic fibers, inhibit the synthesis of melanin (reduce hyperpigmentation) and increase the proliferation rate of keratinocytes and fibroblasts - increase epidermal turnover. It has also been shown that Glycolic Acid can counteract the downregulation of aquaporin-3, which UVB otherwise induces, and thus promote the hydration of the epidermis.
The side effects you can experience with AHA are especially seen when using Glycolic Acid at a high concentration and low pH value – it is a small and effective molecule. As mentioned, pH affects the effects and how easily a molecule with acid-base properties can enter the skin. A more recent ex vivo study with human skin has investigated whether Glycolic Acid at a slightly higher pH value of 4.0, where over half of the molecules are in the corresponding base form, also has the positive effects seen at lower pH.
The concentrations of the compound used in this study were relatively high (between 8 and 25%) and the study showed that you could still see the positive effects in the form of, for example, increased epidermal thickness, increased keratinocyte proliferation and collagen levels.
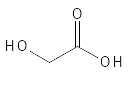
Figure 3: The chemical structure of Glycolic Acid.
LACTIC ACID
Lactic Acid is being used in many products like an exfoliating AHA and also in many cosmetic products to adjust the pH of the finished product - however, a very low concentration is usually used for this. It is found naturally in e.g. acidified milk products and also in the body and is a natural part of the skin's own NMF (Natural Moisturizing Factor). The substance was first isolated in 1780 from sour milk and in 1808 it was discovered that it is also formed in the muscles during exercise. In 1856, it was found that bacteria of the genus Lactobacillus (lactic acid bacteria) synthesize the substance and the chemical structure was determined in 1873. As shown in Figure 4, Lactic Acid is a very small and simple molecule that is very similar to Glycolic Acid. Today, Lactic Acid is produced using bacterial fermentation of carbohydrates or by chemical synthesis – most cosmetics are produced by bacterial fermentation. In pure form, it is a white solid and water-soluble substance with hygroscopic, metal-binding and keratolytic properties. In food, it has the designation E270, where it helps preserve and stabilize goods.
INCREASE THE FIRMNESS OF YOUR SKIN AND AVOID PIGMENTATION
Like, for example, Glycolic Acid, it has been shown that Lactic Acid can increase the thickness and firmness of the epidermis and dermis, act as a moisturizer, reduce wrinkles, acne and also reduce the melanin synthesis by inhibiting the tyrosinase enzyme and thus reducing the pigmentation of the skin. Many studies have been done with Lactic Acid. For example, a study in which 5 or 12% Lactic Acid was used twice a day for 3 months, which demonstrated several of the above-mentioned effects - it was also seen that at 5% there were not the same dermal effects as there were at 12%, but that the clinical and epidermal effects were similar for the two concentrations. An interesting thing about Lactic Acid is that it exists in two stereoisomeric forms, where the spatial structure is slightly different - they are called L-Lactic Acid, which is the version that occurs most in nature, and D-Lactic Acid, which is found in smaller amounts in nature and can be produced by chemical synthesis. A study has shown that L-Lactic Acid is less irritating than D-Lactic Acid and that both work roughly as well as Glycolic Acid in terms of reducing wrinkles - however, Lactic Acid (both forms) was slightly better at providing moisture to the skin compared to Glycolic Acid.
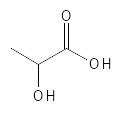
Figure 4: The chemical structure of Lactic Acid.
MALIC ACID
Malic Acid is found, as the name suggests, in apples (Malic from Malum, which means apple in Latin) - and also in, for example, grapes and rhubarb and many other fruits. It is the primary acid in fruits and is found in all living organisms including the human body. The substance was first isolated in 1785 from apple juice and today is often produced by chemical synthesis – efforts are being made to produce it using more sustainable sources and milder techniques such as enzymes or microorganisms.
As can be seen from Figure 5, Malic Acid is a slightly larger molecule compared to Glycolic Acid and Lactic Acid and contains two carboxylic acid groups and an alcohol group which is in position alpha relative to the one carboxylic acid group and in position beta relative to the second carboxylic acid group. Malic Acid is usually classified as an AHA, although in principle it can also be classified as a BHA. Just like the other AHAs, in its pure form it is a white, solid and water-soluble substance with metal-binding and moisturizing properties in addition to being able to exfoliate. It is often used to bind metals and adjust the pH in both cosmetics, cleaning products and food. In foods, where the majority of the Malic Acid produced is used, it has the designation E296 and is used, for example, to improve the taste of both food and drink. In the medical industry, it is used, for example, as a starting material to synthesize medicines, and Malic is also used for other purposes - for example, treatment of dry mouth and in food supplements.
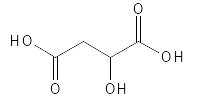
Figure 5: The chemical structure of Malic Acid.
MANDELIC ACID
This substance is found in bitter almonds, where it was just discovered in 1831. Today it is usually produced by chemical synthesis. It is a white, solid and water-soluble substance. As can be seen from Figure 6, it is a slightly larger molecule with a so-called aromatic ring (benzene ring), which means that in addition to being an AHA, it can also be classified as an AMA (Aromatic Hydroxy Acid). The slightly larger molecular size means that it has a little more difficulty getting into the skin and should therefore be a little milder than the smaller AHAs. Compared to the other AHAs mentioned, this one is newer in cosmetics and therefore not quite as many studies have been carried out with it yet. It has been seen that Mandelic Acid has an effect against acne and hyperpigmentation and can improve the firmness and elasticity of the skin. A dermatological in vivo study has shown that treatment with 30% Mandelic Acid for 2-5 minutes had a good effect on acne – this is an example of a professional treatment in a dermatological context, where the highly concentrated product is washed off after a very short time on the skin, as they can otherwise damage the skin. The most common side effects in this context are redness and a burning sensation in the skin. Mandelic Acid has also been used medicinally as an antibacterial agent.
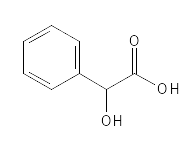
Figure 6: The chemical structure of Mandelic Acid.
TARTARIC ACID
Tartaric Acid is one of the lesser known AHAs. It is found in grapes and quite a few other fruits. Today, it is most often produced by fermentation with yeast organisms or by chemical synthesis. Chemically, it is somewhat similar to Malic Acid in that it also has two carboxylic acid groups and then two alcohol groups, where Malic Acid has one - see figure 7, which also shows that it is twice as large as Glycolic Acid. It is a white, solid, water-soluble substance which can also bind metals and can be found in food under the designation E334, where it is used to adjust the pH, give a particularly sour taste and to help preserve the product. At very high intakes, it can be harmful. In cosmetic contexts, it is believed to have keratolytic and astringent properties and is also used to adjust pH. It is often combined with other AHAs in cosmetic products.
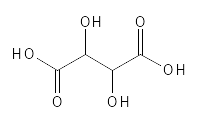
Figure 7: The chemical structure of Tartaric Acid.
BHA – BETA HYDROXY ACIDS
BHA (6*) has, like AHA, been used for many years in both dermatology and cosmetics - especially for the treatment of acne. In general, BHAs are less strong acids compared to AHA and studies show that BHA is milder with fewer side effects and there are no studies that suggest that BHA increases the skin's sensitivity to the sun. As described, BHAs are defined by the fact that there is an alcohol group in the beta position relative to the carboxylic acid group – under this definition there are several substances, e.g. 3-Hydroxybutyric Acid, Tropic Acid and Trethocanic Acid, but in (European) cosmetic contexts Salicylic Acid – and its derivatives – is virtually the only BHA used. So, BHA is almost synonymous with Salicylic Acid in cosmetics in Europe – when you consider that the substances thatcan be classified as both an AHA and a BHA are considered to be AHAs – therefore Salicylic Acid is here the only representative of BHAs.
So, BHA is almost synonymous with Salicylic Acid in cosmetics in Europe – when you consider that the substances that can be classified as both an AHA and a BHA are considered to be AHAs – therefore Salicylic Acid is here the only representative of BHAs.
PUCA - PURE & CARE has a range of products that contain BHA. Find them here.
(6*) BHA is also the abbreviation for Butylated HydroxyAnisole – a food-approved (E320) antioxidant with a possible endocrine-disrupting effect (at very high doses) and which is believed to have a carcinogenic effect – in certain animal experiments, which are not immediately believed to be relevant in relation to humans.
SALICYLIC ACID
Salicylic Acid's exfoliating properties and how it can be produced by chemical synthesis were described in the 1860s, but have been known for thousands of years in the form of extracts from willow trees, which have been used for various skin problems and to relieve pain, fever and inflammation (7*). The name Salicylic Acid comes from the Latin name for willow tree: Salix. Salicylic Acid and several similar substances are also found in e.g. beer, tea, sweet potatoes and nuts. In plants, Salicylic Acid is a phytohormone that is important for plant growth and development.
You can be allergic to Salicylic Acid and similar substances (Salicylates), but this is relatively rare and Salicylic Acid is not classified as an allergen. In case of allergy, it may be necessary to follow a diet with a very low content of salicylates and to avoid products for the skin with salicylates. In addition, there are rare cases of salicylicism, which is the term for poisoning with Salicylic Acid. It can occur if the body absorbs too much of the substance – orally and/or dermally. One should therefore not consume Salicylic Acid in large quantities or use products with a high concentration of Salicylic Acid on large parts of the body or damaged skin. Children are more sensitive than adults. With salicylicism, the central nervous system is affected, so that you can feel nauseous, faint, vomit and, in the worst case, go into a coma and die. It has been seen that it can occur at concentrations of salicylates of around 35 mg/dl in the blood. Other disadvantages of Salicylic Acid are that it can cause serious eye damage and is suspected of harming the unborn child (hazard statement code H361d). It is being discussed whether Salicylic Acid might be a hormone-disrupting substance, which studies are in the process of uncovering. The substance may be slightly irritating to the skin.
THE CHEMICAL PROPERTIES OF SALICYLIC ACID
Salicylic Acid can also be described as a phenolic aromatic acid or AMA, as it contains an aromatic ring (benzene ring) and some believe that Salicylic Acid should be more closely classified as a pseudo-BHA due to the chemical structure (see figure 8) where hydroxy- the group is located on an aromatic ring (benzene ring), which affects its acid properties, as the hydroxy group on the ring structure also has acid properties (can give up the hydrogen atom). In its pure form, Salicylic Acid is a colorless, bitter-tasting solid, which is not very soluble in water, but for example more soluble in alcohol (ethanol), which is important for its effect on the skin compared to AHAs, as it makes it easier for it to come into contact with the intercellular fats and get down the skin's pores and sebaceous glands, where the accumulation of corneocytes can cause problems and contribute to, for example, acne. Salicylic Acid is often used precisely for the treatment of acne, as it can act on several factors in the development of acne: Salicylic Acid can reduce the level of sebum, it has anti-microbial and anti-inflammatory effects and it is keratolytic – can exfoliate the skin - and due to its more fat-soluble properties, it can also have a comedolytic effect, which means it can eliminate comedones (blackheads, which are accumulations of sebum in sebaceous glands) and inhibit the formation of new ones.
SALICYLIC ACID IN SKINCARE
In addition, studies have shown that Salicylic Acid, like some of the AHAs, can reduce hyperpigmentation and wrinkles. Due to its anti-microbial – including anti-fungal – effect, Salicylic Acid can also be used as a preservative and against dandruff (8*). As a preservative, it is permitted to use up to 0.5% in the EU. In hair products that are washed off, up to 3.0% may be used and in other products up to 2.0% (9*) - however, it may not be used in products for children (apart from shampoos) and if a product containing Salicylic Acid could be considered to be used on children there must be a warning not to use it on children under 3 years of age.
The detailed mechanisms behind all these effects are not fully established, but it is believed that, like AHA, Salicylic Acid can loosen the cohesion between the corneocytes and penetrate and loosen the intercellular lipids more easily than the AHAs. Regarding pH, a study has shown that the exfoliating effect of Salicylic Acid is apparently not particularly affected by the product's pH, thus equally good effects were seen at pH 3.12 and 6.5, and in addition it did not induce a stinging sensation at pH 6.5 in contrast to the product with pH 3.12. How easily Salicylic Acid can get into the skin seems to be affected by the product's composition, pH, skin quality and how the product is used. In in vivo studies, skin absorption of approximately 20-60% has been observed. The importance of the product's composition in relation to skin absorption is shown, for example, in a study where 1.5% Salicylic Acid was used and two formulations were compared, one of which contained a raw material that was found to be able to increase the speed with which Salicylic Acid entered the skin. The same study showed a significant effect on akne for most subjects after 4 weeks. Another study using 1% of Sodium Salicylate (the sodium salt of Salicylic Acid) showed a significant reduction in wrinkles and an increase in Collagen I levels.
In addition to cosmetic use, Salicylic Acid is also used medicinally for the treatment of warts and skin diseases such as ichthyosis and psoriasis, in veterinary products as an anti-fungal and antiseptic substance, in food to preserve and it is also used as a starting compound for producing e.g. colors and the medicine Acetylsalicylic acid.
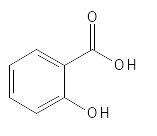
Figure 8: The Chemical Structure of Salicylic Acid.
(7*) Salicylic Acid is the starting material for acetylsalicylic acid, which is a commonly used antipyretic, pain reliever and anti-inflammatory drug.
(8*) Dandruff is often associated with an imbalance in the microbial flora – especially fungi of the genus Malassezia.
(9*) In 2018, SCCS (the EU's Scientific Committee of Consumer Safety) assessed that up to 0.5% is safe for body lotion, eye shadow, mascara, eyeliner, lip products and roll-on deodorants. This assessment will possibly be implemented in cosmetic legislation.
PHA – POLY HYDROXY ACIDS
PHA is often referred to as the next generation of HAs. There aren't that many studies with them yet, but some studies suggest that they can work very similar to AHAs and that they are milder with fewer side effects. The classification of a PHA is, as mentioned, that in addition to the carboxylic acid group, the molecule contains a minimum of two (often more) hydroxy groups and usually one of them is in the alpha position in relation to the carboxylic acid group. A subgroup of PHA are the so-called PHBAs, which stand for Poly Hydroxy Bionic Acid (sometimes called aldonic acid), which are PHAs that have a sugar molecule in the structure. The most known and used PHAs are:
- Gluconolactone
- Lactionic Acid
- Maltobionic Acid
They are mild exfoliants and most are also antioxidants and moisturizers. As seen from the chemical structures in the following sections, PHAs are slightly larger molecules that will have a harder time penetrating the skin, which probably contributes to their mildness. Just like AHAs, they are fairly water-soluble. Their effect on the skin probably comes from a combination of their mild exfoliating properties, as well as the moisturizing and antioxidant properties. It is believed that part of the aging that takes place is due to oxidative stress and therefore antioxidants are believed to have an anti-aging effect. In the following, the three representatives of the PHA group will be presented.
PUCA - PURE & CARE has a number of products that contain PHA. See them all here.
GLUCONOLACTONE (GLUCONIC ACID)
Glunonolactone is also sometimes called Glucono Delta-Lactone (GDL) and as the name indicates, this molecule is not an acid, but a so-called lactone - a cyclic carboxyl ester. As seen in figure 9, Gluconolactone is a ring-shaped structure which is in equilibrium with the "open" chain structure - Gluconic Acid - when water is present. Water will react with some of the Gluconolactone molecules and hydrolyze them so that the ring opens and Gluconic Acid with the HA structures is formed. And it can also go the other way so that you can convert Gluconic Acid into Gluconolactone by removing water. Thus, in aqueous environments you will have a mixture of Gluconolactone and Gluconic Acid, and the warmer and higher the pH, the more will be in Gluconic Acid form (and at pH above approx. 4 this will be partly in the form of its corresponding base, where hydrogen- the atom is released). As can also be seen from Figure 9, Gluconic Acid contains 5 alcohol groups (one in each of the positions alpha, beta, gamma, delta and epsilon).
Gluconolactone-Gluconic Acid is found naturally in e.g. fruits, honey and wine and in the human body and other organisms. The two substances can be produced from each other (by removing or adding water) and they can, for example, be produced by chemical synthesis and microbial fermentation of glucose. In pure form, Gluconolactone is an odorless, white, water-soluble solid.
It is used in many different contexts, e.g. in the construction industry for cement, for cleaning agents, in food, where it has the designation E575 and is used to adjust pH and bind metals and it is used in cosmetics. In addition to being able to adjust pH and bind metals, it is also an antioxidant, moisturizer and it can exfoliate the skin (probably in the form of Gluconic Acid). In studies, Gluconolactone has been shown to improve skin moisture, fine lines, the skin barrier and even the skin tone. For example, in a 12-week study, where it was shown that the significant and positive anti-aging effects of Gluconolactone were comparable to Glycolic Acid (using almost the same concentrations and formulations with the same pH value of approx. 3.7) and that Gluconolactone was milder in the form of less redness, burning and stinging sensation in the subjects.
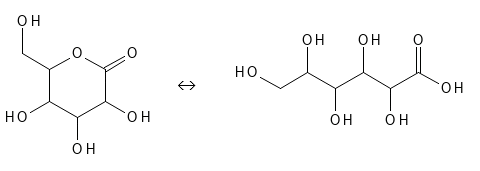
Figure 9: The chemical structure of Gluconolactone which is in balance with the acid form, Gluconic Acid.
LACTOBIONIC ACID
Lactobionic Acid belongs to the subcategory of PHBA - Poly Hydroxy Bionic Acid, since as Figure 10 shows, it contains a sugar molecule (the 6-membered ring called galactose) and the other part of the molecule is Gluconic Acid. It can also be noted in Figure 10 that some of the bonds are shown as dashed or thicker wedge-shaped lines. This is to show the spatial structure of the molecule (dotted wedge-shaped bonds point away from the viewer and thicker wedge-shaped bonds point towards from the viewer) as this is where the differences between Lactobionic Acid and Maltobionic Acid lie - compare figure 10 and 11. Such stereoisomerism can be important for the biochemical effects of substances.
(10*) In some of the other chemical structures, their stereochemical structure could also have been shown. For the sake of simplicity, this has been omitted.
Like the other PHAs, in addition to exfoliating, Lactobionic Acid can also bind metals and act as an antioxidant and moisturizer. In its pure form, it is syrup-like. It can be produced by microbial fermentation and by chemical synthesis. In addition to cosmetics, Lactobionic Acid is also used in food products, where it is used to e.g. stabilize and improve the taste - here it is designated E399 (more specifically, it is the Calcium salt of Lactobionic Acid's corresponding base: Calcium Lactobionate).
Since it is a relatively new substance in cosmetic contexts, not many studies have yet been done with it, but it has shown, for example, in a 12-week study using an 8% cream to be able to increase the skin's thickness and improve the skin's structure and overall have an anti-aging effect.
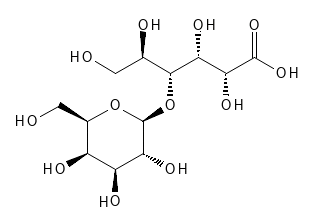
Figure 10: The Chemical (and Stereochemical) Structure of Lactobionic Acid. (10*)
MALTOBIONIC ACID
Like Lactobionic Acid, Maltobionic Acid belongs to the subcategory of PHBA - Poly Hydroxy Bionic Acid and can be produced via the same methods. The structures of these two stereoisomeric substances differ only in the three-dimensional structure - see figures 10 and 11. The two substances have many of the same properties: moisturizing, antioxidant, exfoliating and binding metals. Like Lactobionic Acid, it is a relatively new substance that has been shown to have anti-aging effects. They can, for example, reduce fine lines, improve the skin's structure, reduce dryness and give a more even skin tone. In in vitro experiments, it has been shown to be able to increase epidermal thickness, provide a more compact stratum corneum, reduce UV-induced lipid oxidation (act as an antioxidant), inhibit hyperpigmentation after sun exposure and reduce the breakdown of collagen by inhibiting MMP enzymes, which otherwise breaks down the skin's collagen. In vivo , this PHBA has also been shown to be gentle on the skin.
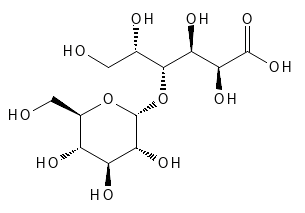
Figure 11: The Chemical (and Stereochemical) Structure of Maltobionic Acid.
SOURCES:
Arif T. Salicylic acid as a peeling agent: a comprehensive review. Clinical Cosmetic and Investigational Dermatology. 2015; 8:455-461.
Audina, Mia. A review on anti-aging properties of polyhydroxy acids. World Journal of Pharmaceutical Research. 2021; 10. pp. 137-141.
Babilas, P.; Knie, U.; & Abels, C. Cosmetic and dermatologic use of alpha hydroxy acids. Journal der Deutschen Dermatologischen Gesellschaft = Journal of the German Society of Dermatology: JDDG. 2012; 10(7), 488–491.
Chen, X.; Wang, S.; Yang, M.: & Li, L. Chemical peels for acne vulgaris: a systematic review of randomised controlled trials. BMJ open. 2018; 8(4), e019607.
Ditre, C. M; Chilek, K. D. Exfoliants, Moisturizers and More: AHAs,BHAs, and PHAs – Chapter 16 in Procedures in Cosmetic Dermatology Series Cosmeceuticals, 2e. 2015. Accessed 7th September 2022.
Edison, B.L.; Green, B.A.; Wildnauer, R.H.; Sigler, M.L. A polyhydroxy acid skin care regimen provides antiaging effects comparable to an alpha-hydroxyacid regimen. Cutis. 2004 Feb; 73 (2 Suppl):14-17.
Fabbrocini, G.; De Padova, M.P. & Tosti, A. Glycolic acid. & Salicylic acid. In: Tosti, A.; Grimes, P.E. & De Padova, M.P., eds. Color Atlas of Chemical Peels. 2. Edition. Springer, Berlin: Heidelberg; 2012.
Fiume M. M. Alpha Hydroxy Acids. International journal of toxicology. 2017; 36 (5 suppl 2), 15S–21S.
Garg, V. K.; Sinha, S.; & Sarkar, R. Glycolic acid peels versus salicylic-mandelic acid peels in active acne vulgaris and post-acne scarring and hyperpigmentation: a comparative study. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2009; 35(1), 59–65.
Green, B. A.; Briden, E. PHAs and Bionic Acids: Next Generation Hydroxy Acids– Kapitel 33 i Procedures in Cosmetic Dermatology Series Cosmeceuticals, 2e. 2015. Lokaliseret 7. september 2022.
Grimes, P. E.; Green, B. A.; Wildnauer, R. H.; & Edison, B. L. The use of polyhydroxy acids (PHAs) in photoaged skin. Cutis. 2004; 73(2 Suppl), 3–13.
Jacobs, S.W.; & Culbertson, E.J. Effects of Topical Mandelic Acid Treatment on Facial Skin Viscoelasticity. Facial Plastic Surgery. 2018; 34, 651–656.
Jartarkar, R.; Mallikarjun, D.S.; Gangadhar, D.; & Manjunatha, D.K. Mandelic acid chemical peel in Acne vulgaris: A boon or a bane? IOSR Journal of Dental and Medical Sciences. 2015; 14, Issue 5 ver VII. 32-35.
Kornhauser, A.; Coelho, S. G.; & Hearing, V. J. Applications of hydroxy acids: classification, mechanisms, and photoactivity. Clinical, cosmetic and investigational dermatology. 2010; 3, 135–142.
Kövilein, A.; Kubisch, C.; Cai, L.; & Ochsenreither, K. Malic acid production from renewables: a review. Journal of Chemical Technology & Biotechnology. 2020; 95: 513-526.
Kraeling, M.E.; & Bronaugh, R.L. In vitro percutaneous absorption of alpha hydroxy acids in human skin. Journal of the society of cosmetic chemists. 1997; 48, 187-197.
Merinville, E.; Byrne, A.J.; Rawlings, A.V.; Muggleton, A.J.; & Laloeuf, A.C. Original Contribution: Three clinical studies showing the anti-aging benefits of sodium salicylate in human skin. Journal of Cosmetic Dermatology. 2010; 9: 174-184
Merinville, E.; Laloeuf, A.; Moran, G.; Jalby, O.;& Rawlings, A.V, Exfoliation for sensitive skin with neutralized salicylic acid?. International Journal of Cosmetic Science. 2009; 31: 243-244.
Narda, M.; Trullas, C.; Brown, A.; Piquero-Casals, J.; Granger, C.; & Fabbrocini, G. Glycolic acid adjusted to pH 4 stimulates collagen production and epidermal renewal without affecting levels of proinflammatory TNF-alpha in human skin explants. Journal of cosmetic dermatology. 2021; 20(2), 513–521.
PubChem Sketcher V2.4. Accessed 27th September 2022: