Lipids-Oils
Lipids and oils
Lipids is a broad term for a not entirely well-defined group of very diverse molecules which e.g. include triacylglycerides, waxes, phospholipids, glycolipids and steroids – fatty compounds, many of which are vital to life on earth.
Oils are complex mixtures consisting of lipids and it is the composition of these lipids that determines the properties of the mixture. Oils are liquid at room temperature, while butters and fats are often used for the fatty compositions which are more solid at room temperature.
Here, the term "oil" will be used broadly. Oils can be categorized according to many different parameters. For example based on:
- Origin: Animal (e.g. from fish, birds, mammals and insects), vegetable (from plants and algae), petrochemical (from mineral oil) and chemically synthesized (e.g. silicone oils).
- Production method: Mechanical pressing, extraction with solvents and possible subsequent refining in various ways.
- Physical properties such as how volatile they are: The volatile essential oils and the non-volatile oils.
- Chemical properties such as molecular size and fatty acid composition: Whether it is primarily unsaturated or saturated fatty acids that are included. Also, the composition of the oil regarding the saponifiable and unsaponifiable substances is often of interest.
Here the focus will be on vegetable non-volatile oils, which are important in many respects – both for the health of the whole body and in skin and hair care.
PUCA PURE & CARE uses many different vegetable oils and substances derived from vegetable oils in its products. Here are just some of them:
Butyrospermum Parkii Oil (shea oil), Macadamia Ternifolia Seed Oil (macadamia oil), Persea Gratissima Oil (avocado oil), Simmondsia Chinensis Seed Oil (jojoba oil), Cocos Nucifera Oil (coconut oil), Olea Europaea Oil Unsaponifiables (the unsaponifiable part of olive oil), Caprylic/Capric Triglyceride (tri(acyl)glycerides made with the fatty acids Caprylic Acid and Capric acid, which are usually extracted from coconut oil), Squalane, Argania Spinosa Kernel Oil (argan oil), Tocopheryl Acetate (vitamin E acetate ) and also a few selected essential (volatile) oils: Melaleuca Alternifolia Leaf Oil (tea tree oil), Lavandula Hybrida Oil (lavender oil) and Citrus Aurantium Dulcis Oil (orange oil).
Lipids - a group of very different compounds
For many years, lipids were considered scientifically less interesting and that they primarily fulfilled two important purposes: providing energy and building cell membranes. It wasn’t until around the 1950s that people began to discover the importance of lipids in many other respects - and it has since been discovered that there are many lipids with unique biological functions that differ from being a source of energy and simple structural units.
Lipids together with polysaccharides, proteins and nucleic acids make up the four main groups of macromolecules; but unlike the other three groups, there is no internationally accepted definition of what lipids are. Often, lipids are described as substances that are insoluble in water but soluble in non-polar (organic) solvents – i.e. a definition based on physico-chemical properties in contrast to the definition of the other macromolecules, which is based on the chemical structure of the molecules. Such a definition of lipids includes an enormously broad group of substances and, in a way, may also exclude substances which in science are generally considered to be lipids. Many lipids are amphiphilic, meaning that they contain a part that prefers to be in water and another part that prefers to be in a more non-polar organic solvent.
Several research groups have tried to give others clearer definitions and divide lipids into different groups. One of the simpler groupings is in the "simple lipids", which upon hydrolysis result in a maximum of two molecule types and the "complex lipids", which upon hydrolysis result in a minimum of three molecule types. Since 2005, other researchers have worked to spread a different definition of lipids based on which molecules they are made up of (a definition more similar to the definitions for the other macromolecules) and from there created 8 categories of lipids and developed a more systematic nomenclature for the molecules. The 8 categories are: Fatty acyls, glycerol-lipids, glycerophospho-lipids, sphingo-lipids, sterol-lipids, prenol lipids, saccharo-lipids, and polyketides. Each category is further divided into classes and subclasses. Here is a brief description of the 8 categories1: Fatty acyls, glycerol-lipids, glycerophospho-lipids, sphingo-lipids, sterol-lipids, prenol lipids, saccharo-lipids, and polyketides. Each category is further divided into classes and subclasses. Here is a brief description of the 8 categories:
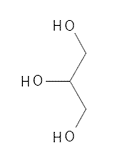
Figure 1 The chemical structure of Glycerin (glycerol).
It is not known exactly how many different lipids exist in nature, but there are believed to be over 200,000, many of which belong to the category of prenol lipids and polyketides. Some lipids are specific to certain animal and plant groups, and some are widely distributed – e.g. triacylglycerides. Triacylglycerides are found to a greater or lesser extent in most plants and animals and are also the lipid class that makes up the vast majority of the fat humans consume via food. The fat that animals and humans accumulate on the body as energy storage and insulation is found in the form of triacylglycerides. In general, vegetable non-volatile oils consist of over 90% triacylglycerides and therefore this lipid class will be reviewed more thoroughly here.
Triacylglycerides consist of a glycerol molecule to which three fatty acids are attached with ester bonds - see Figure 2. A glycerol molecule consists of three carbon atoms in a row and on each of these there is an alcohol group (-OH ) – see Figure 1. A fatty acid consists of a chain of carbon atoms, which at one end has a carboxylic acid group (-COOH) – see Figure 3 and Figure 4. To form an ester bond between the glycerol molecule and the fatty acid the alcohol group in glycerol reacts with the carboxylic acid group on the fatty acid and water is released. The reverse reaction where the ester bond is broken is called hydrolysis and it happens, for example, when you make classic (solid) soap from fat and a base such as Sodium Hydroxide.
- The category of fatty acyls contains, for example, fatty acids, fatty alcohols and waxes, which consist of a fatty acid and a fatty alcohol bound together via an ester bond.
- The category Glycerophospho-lipids are often simply called phospholipids and these are a key component of cell membranes. These molecules consist of a glycerol (Glycerin) unit, on which there are two hydrophobic fatty acids and a hydrophilic phosphate group. They are thus amphiphilic molecules.
- The category Glycero-lipids contains the very important group of tri-acyl-glycerides, which are often simply called triglycerides (see Figure 2) and also di-acyl-glycerides (diglycerides) and mono-acyl-glycerides (monoglycerides). These molecules consist of a glycerol unit (Glycerin - see Figure 1) on which are respectively three, two or one fatty acid. Triacylglycerides will be reviewed in more detail as these molecules make up the vast majority of all vegetable oils.
- The category of sphingo-lipids includes, among other compounds the sphingo-lipids, which are involved in the construction of cell membranes and ceramides, some of which are very important for the properties of the skin.
- The category of sterol lipids contains, for example, cholesterol, which is also part of cell membranes, and which is the starting point for the biosynthesis of the steroid hormones and bile salts and vitamin D – thus a very important molecule. Phytosterols are found in plants which are very similar.
- The category of prenol lipids includes, among other compounds isoprenoids such as squalene, retinoids, tocopherols2 and terpenes such as the carotenoids and some of the substances that make up essential oils.
- The category of saccharo-lipids is a lesser-known group of amphiphilic substances consisting of fatty acids bound directly to sugar units, which are known from, among other species, some bacteria.
- The category of polyketides contains several classes and subclasses and a great many different substances – several of which have antimicrobial effects, and some are toxins. The large class of flavonoids also belongs to the polyketides.
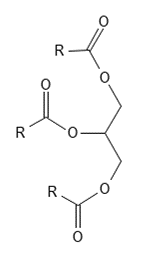
Figure 2 The basic chemical structure of triacylglycerides - a glycerol molecule with three fatty acids linked via ester bonds. R represents fatty acid chains.
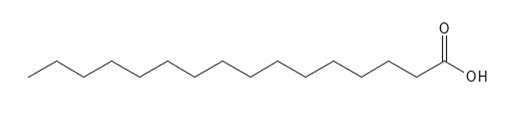
Figure 3 The chemical structure of the saturated fatty acid Palmitic acid (C16:0).
1You can read more about the different types of lipids here: https://www.lipidmaps.org/
2Read more about retinoids and Vitamin E in the description on this website.
The classification of fatty acids
The three fatty acids in a triacylglyceride molecule are typically two or three different fatty acids3 (see Figure 6) and it is these fatty acids that determine which properties the molecule has and thus also which properties a mixture such as oils consisting of triacylglycerides has. Oils usually also contain a small amount of other lipids, which also contribute to the properties of the oil.
Fatty acids in nature are most often unbranched (consist of one carbon chain) and most often have an even number of carbon atoms in the chain. Chain length (how many carbon atoms are in the chain) can vary a lot and most often they are divided into short chains, which consist of less than 6 carbon atoms; medium chain, which consists of 6-12 carbon atoms; long chain, which consists of 13-21 carbon atoms and the very long chain, which consists of more than 22 carbon atoms. The bonds between the C atoms in the chain are primarily single bonds, but they can also be double bonds, giving an unsaturated fatty acid.
Fatty acids are thus divided into saturated, which only contain single bonds, and unsaturated, which contain at least one double bond between two carbon atoms in the chain. The unsaturated fatty acids are further divided into the monounsaturated (MUFA4), which contain exactly one double bond, and the polyunsaturated (PUFA5), which contain more than one double bond. These double bonds can either be cis or trans configuration, which means that the carbon atoms next to the two carbon atoms that have a double bond between them point in the same (cis) or in the opposite (trans) direction.
In nature, most unsaturated fatty acids have cis configuration, but by e.g. hydrogenation6 of unsaturated fatty acids, trans fatty acids can be formed - Figure 4 and Figure 5 are examples of two monounsaturated fatty acids with cis and trans configuration respectively.
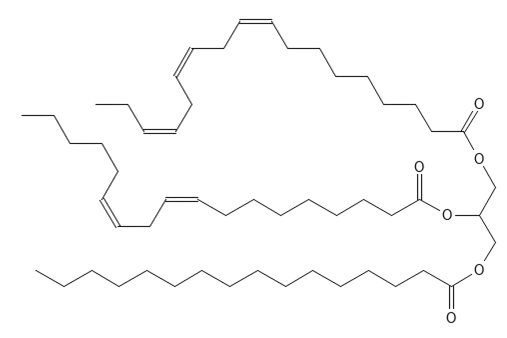
Figure 4 The chemical structure of the monounsaturated omega-9 fatty acid Oleic acid (C18:1) with a double bond in the cis configuration.
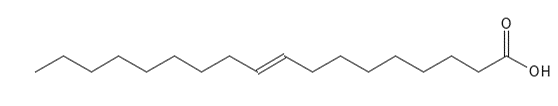
Figure 5 The chemical structure of the monounsaturated fatty acid Elaidic acid (C18:1) with a double bond in trans configuration.
3The location of the three fatty acids in positions 1, 2, and 3 on the glycerol molecule also has a significance, but this has not been studied much.
4MUFA is the abbreviation for MonoUnsaturated Fatty Acid.
5PUFA is the abbreviation for PolyUnsaturated Fatty Acid.
6Hydrogenation is a chemical process in which hydrogen is used to convert, for example, double bonds into single bonds.
The location of double bonds
The location of double bonds in unsaturated fatty acids is of great importance for the biological properties.
The term omega-x is often used to describe where the last double bond in the fatty acid chain is located. To describe the position of a double bond, the carbon atoms are numbered from the methyl end of the chain, which is in contrast to how carbon atoms are normally numbered in the systematic IUPAC nomenclature7, where the carbon atoms are numbered from the carboxylic acid end.
If the double bond is between carbons 3 and 4 then it is an omega-3 fatty acid and if a double bond is located between carbons 6 and 7 it is an omega-6 fatty acid. It is the double bond closest to the methyl end that determines whether it is called an omega-3 or omega-9 fatty acid.
Thus, a fatty acid may well have a double bond between both carbons 3 and 4 and between carbons 6 and 7 and between carbons 9 and 10 (this is the case for the fatty acid α-Linolenic acid; see Figure 6); but it is referred to as an omega-3 fatty acid. In addition to the systematic IUPAC name, there are also trivial names for the most common fatty acids. Below is a list of some of the most common fatty acids with an indication of whether they are saturated, monounsaturated or polyunsaturated fatty acids, the trivial name and number of carbon atoms in the chain (e.g. C12), as well as the number of double bonds and, for unsaturated ones, which omega designation the fatty acid has.
The last two in the list - the omega-3 fatty acids EPA and DHA - are mainly found in fish, which get it from the microalgae they eat. They have been shown to be of great importance to human health. EPA is, for example, a precursor for the formation of some prostaglandins, which are a group of special signaling substances with decisive importance for e.g. blood clotting ability, pain and inflammation.
DHA is particularly important for the eye and brain – brain tissue consists of 60% lipids of which approx. 25% is DHA (as part of Glycerophospho-lipids). The ratio between EPA and DHA has been shown to be important for health, and so has the ratio between ingested omega-3 and -6 fatty acids.
For humans, there are only two essential fatty acids that the body cannot biosynthesize itself and must therefore be consumed through food: Linoleic acid (a C18 polyunsaturated omega-6 fatty acid) and α-Linolenic acid (a C18 polyunsaturated omega-3 fatty acid. These two essential fatty acids are extremely important – for example, Linoleic acid is an important component of many ceramides in the skin – and they are also precursor for the body's biosynthesis of C20 and C22 polyunsaturated fatty acids such as Arachidonic acid, EPA and DHA, each of which is of great importance for the body.
Saturated fatty acids
- Caproic acid – C6:0
- Caprylic acid – C8:0
- Capric acid – C10:0
- Lauric acid – C12:0
- Myristic acid – C14:0
- Palmitic acid – C16:0
- Stearic acid – C18:0
- Arachidic acid – C20:0
- Behenic acid – C22:0
Monounsaturated fatty acids
- Palmitoleic acid – C16:1; Omega-7
- Oleic acid – C18:1; Omega-9
- Erucic acid – C22:1; Omega-9
Polyunsaturated fatty acids
- Linoleic acid – C18:2; Omega-6
- α-Linolenic acid – C18:3; Omega-3
- γ-Linolenic acid – C18:3; Omega-6
- Arachidonic acid – C20:4; Omega-6
- Eicosapentaenoic acid (EPA) – C20:5; Omega-3
- Docosahexaenoic acid (DHA) – C22:6; Omega-3

Figure 6 The chemical structure of a triacylglyceride with three different fatty acids in the three positions on the glycerol unit. In place 1 (top) is the essential omega-3 fatty acid α-Linolenic acid (C18:3), in place 2 (middle) is the essential omega-6 fatty acid, Linoleic acid (C18:2) and in place 3 (bottom) is the saturated fatty acid Palmitic acid (C16:0).
7IUPAC is the abbreviation for International Union of Pure and Applied Chemistry. An international organization, which among other things has prepared nomenclature for chemical substances.
Oils
Oils from plants have been used for thousands of years in many cultures. For example, evidence has been found for the production of olive oil from around 6000 B.C. The oils are believed to have been used for e.g. food and combustion in e.g. oil lamps and later for the production of e.g. soap, perfumes and lubricants. Today, vegetable oils are used for many different purposes; e.g. for food, animal feed, cosmetics, paint and for the production of many other very different substances such as detergents (detergents), emulsifiers, biofuel and lubricants.
Most vegetable oils are extracted from the seeds of the plant and some from the fruit. Microalgae are one of the newer sources of vegetable oils, some of which contain special lipids. Palm oil and soybean oil are the two oils that are produced the most worldwide, followed by rapeseed oil, sunflower oil, palm kernel oil, peanut oil, cottonseed oil, olive oil, corn oil, coconut oil and many more in smaller quantities. Soy, grape, cocoa, sunflower, palm kernel and safflower oil are examples of seed oils, while olive, palm, avocado and coconut oil are examples of oils from plant fruits.
For the extraction of vegetable non-volatile oils8, such as those mentioned above, different methods can be used. In general, they can be divided into mechanical and solvent extraction and often several methods are used to extract as much oil as possible from the plant material. You will often start by pressing oil out mechanically, which yields what is often called cold-pressed oil. Heat can also be applied to squeeze more oil out of plant material. The plant material will often still contain some oil, which can then be extracted with solvents such as n-Hexane (which is subsequently removed). Some oils are extracted by CO2 extraction.
The oils obtained via these methods will often undergo several different purification and refining processes, all of which change and usually remove some components of the oil. Some of these processes are less specific so that you run the risk of removing both unwanted and desired components. Examples of purification and refining processes are steam distillation, which can remove odors (deodorization) and reduce the content of Tocopherol and free fatty acids; degumming, which can remove free fatty acids and phospholipids, and special filters or other physical methods, which can remove dyes and waxes. These processes are generally carried out to improve the quality and shelf life of the oil. In some respects, however, it is not desirable that e.g. Tocopherol is removed and therefore methods are gradually being developed which are more selective in which substances they remove - examples of such newer methods are molecular distillation and supercritical CO2 fractionation.
The oils produced are mixtures of many different lipids and the composition can vary, as the plants' biosynthesis processes can be affected by, for example, climate, maturity, and treatment of the plant material. In general, the oils primarily consist of triacylglycerides and may also contain smaller amounts of, for example, free fatty acids, sterols, phospholipids, waxes, squalene, phenols and vitamins such as tocopherol. The lipids are often divided into the saponifiable ones, which normally make up approx. 99%, and the unsaponifiable lipids, which make up the last approx. 1% of the oil.
The principle of saponification, where classic (typically solid) soap is made from oils, is that you treat the oil with a base such as Sodium Hydroxide, which causes the ester bonds to be broken, so that fatty acid salts (soaps) are formed and the alcohol part is released, which for triacylglycerides is glycerol (Glycerin) and for waxes is fatty alcohol. Thus, the saponifiable part of oils are the lipids that contain ester bonds such as triacylglycerides, phospholipids and waxes and the unsaponifiable part are lipids such as sterols, squalene, phenols, carotenoids and tocopherols.
It is generally the unsaponifiable lipids that give the oils characteristics such as color, fragrance and taste and certain bio-chemical properties are assigned to them.
Other physical properties such as how the oil feels (sensory) and whether it is liquid or solid (melting point) are primarily determined by the fatty acid composition of the oil's triacylglycerides. Oils are often divided into sensory fatty and dry/light oils, of which the dry/light oils generally contain the most omega-3 and omega-6 fatty acids. With regard to the oil's melting point, it generally applies that the more double bonds in fatty acid chains, the lower the melting point. Thus, most vegetable oils are liquid at room temperature (i.e. have a melting point below room temperature) as they contain a large proportion of polyunsaturated fatty acids; while vegetable butter and animal fat are generally solid at room temperature (i.e. have a melting point above room temperature), as they primarily contain saturated fatty acids.9. The fatty acid composition, as mentioned, also plays a significant role in the biological properties of the oil.
8The volatile essential oils, which are often extracted from plants, are generally produced by steam distillation and some (primarily citrus oils) by mechanical pressing.
9You can read more about the fatty acid composition of a number of different vegetable oils in the following article: Vegetable Butters and Oils as Therapeutically and Cosmetically Active Ingredients for Dermal Use: A Review of Clinical Studies. Written by Poljšak, N.; & Kočevar Glavač, N. in the journal Frontiers in pharmacology. 2022; 13, 868461.
Lipids and the body
Lipids, like carbohydrates, proteins and nucleic acids (e.g. DNA), are vital for the human body. The importance of lipids to the body is a very large subject which will only be briefly described here.
Lipids perform many functions in the body; for example, they (primarily triacylglycerides) are an important source of energy, just like carbohydrates and proteins, and triacylglycerides are the body's most efficient way to store energy and also insulate the body and organs. Lipids - primarily in the form of phospholipids, sphingolipids and sterols make up the majority of each cell membrane, which is usually about 5 nm thick and inside each cell all organelles10 are also surrounded by a membrane consisting primarily of lipids.
The membranes are generally lipid bilayers, which means that there are two layers of lipids on top of each other. Many of the lipids that form the cell membrane – primarily the phospholipids and the sphingolipids – are amphiphilic molecules, which face such that their hydrophilic end points outward from and inward into the cell, which is surrounded by and contains aqueous fluid; while their hydrophobic part (the fatty acid chains) point towards the center of the lipid bilayer and interact with the second layer of lipids in the membrane. Likewise, in the cell membrane there are also proteins which have their more hydrophobic part to interact with the center of the cell membrane and their more hydrophilic part sticks out from or into the cell. The sterols in membranes, which in mammals are primarily cholesterol, contributes to the membrane having the right fluidity and permeability. Membranes in plants have similar sterols – phytosterols – in their cell membranes. Each lipid layer in the cell's many membranes has its own dynamic composition of lipids, which is important for the functions of the membrane.
Some lipids function as hormones – for example, steroid hormones belong to the group of sterol lipids – and other lipids are signal molecules or precursors to signal molecules. Prostaglandins, which are important signaling molecules in all body tissues, are also lipids – they are biosynthesized from the fatty acid Arachidonic acid.
The vitamins A, D, E and K also belong to the group of lipids and the transport of these and other lipids in the blood takes place with special aggregates called lipoproteins, which consist of lipids and proteins. In addition to the lipids that are ingested via food, which after ingestion undergo a number of processes in order to be absorbed and distributed in the body's tissues and can be modified in various ways along the way, the body can also biosynthesize many lipids itself – for example from glucose.
A number of different diseases such as certain cardiovascular diseases and diabetes have an imbalance in the metabolism of lipids as part of their cause and therefore research into the function of lipids for the body is also an important topic in relation to disease and health.
For plants, lipids are similarly important components of cell membranes, are signaling substances and act as an energy reserve (e.g. in the seeds). In addition, plants often have a thin layer of wax on the surface, which helps to protect and provide waterproofing.
10Organelle is the name for the cell's internal structures ("organs"), which are surrounded by a membrane and which perform various functions. A few examples of organelles are the cell nucleus, which contains DNA, and the mitochondria, which produce most of the cell's energy (ATP).
Lipids and the skin
Lipids also play particularly important roles for the skin – the body's largest organ, which i.a. protects the body from external factors, excretes certain waste products, regulates body temperature, is a sensory organ and hosts the skin's important microbiome. All these functions require many different components in the skin, of which lipids in particular help to provide the protective skin barrier and retain moisture in the skin.
The skin consists of several layers11 - the innermost is the subcutis/hypodermis, the middle is the dermis and the outermost is the epidermis, which consists of several layers. In relation to the skin barrier, it is especially the outermost 10-30 um thick layer of the epidermis, the Stratum corneum, that is important. The stratum corneum contains 15-25 layers of primarily dead, flat skin cells called corneocytes – these cells are embedded in an intercellular lipid-rich matrix with specially organized lipids, which are a crucial element of the skin barrier. These intercellular lipids make up about 15% of the weight of the stratum corneum and are primarily ceramides (about 50%), cholesterol (25-30%) free fatty acids (10-15%); cholesterol esters (about 10%), cholesterol sulfate (2-5%) and only very little phospholipids, which is in contrast to the other layers of the epidermis and dermis, in which phospholipids make up a considerable part of the lipids. Variation in lipid composition of stratum corneum intercellular lipids occurs, for example, between different skin areas on the body. In addition, the lipid composition changes with age, how much the skin is exposed to sunlight, climate and the density of sebum glands in the skin and other factors.
There are 9 different classes of ceramides in the human stratum corneum, which are mainly biosynthesized by the keratinocytes in the stratum granulosum layer of the epidermis. The essential fatty acid Linoleic acid is a key component for some of the ceramides and so is the enzyme family of sphingomyelinases, which catalyze the conversion of sphingomyelin to ceramide and phosphorylcholine, whose reduction in activity is associated with aging of the skin. The free fatty acids in the stratum corneum are mainly long-chain C16-C26 fatty acids - of which Palmitic acid (C16:0) makes up about 10% - and some have an odd number of C atoms. The cholesterol synthesis is very complex and involves, among other things, squalene, which is cyclized to the characteristic sterol structure consisting of 4 ring structures, with an alcohol group on one ring. On this ring, for example, a fatty acid can be added so that you get a cholesterol ester, or it can be converted into a sulfate group so that you get cholesterol sulfate.
In addition to the intercellular lipids in the stratum corneum, sebum from the skin's sebaceous glands – and lipids from the skin's microbiome – also contribute to the lipid composition of the skin surface and the skin barrier. The sebaceous glands have a connection with and outlet in the hair follicles, which are located in the dermis, so that sebum is released to the surface of the skin via the channel in the hair follicle in which the hair strand is. The lipid composition of sebum varies, just like the intracellular lipids, between different skin areas, age, sex, etc. The largest component is triacylglycerides (about 40-45%), followed by wax esters (about 25%), squalene (about 12%), free fatty acids (10-15%), cholesterol and cholesterol esters (about 4%) and diacylglycerides (approx. 2%) and also a small amount of glycerin and tocopherols. The triacylglycerides and the free fatty acids in sebum typically have a chain length of C12-C30 and some of them have anti-microbial effects and many of them are unsaturated. Squalene is a special polyunsaturated hydrocarbon12that belongs to the prenol lipids and is a lipid that is very specific to sebum and has shown several different interesting biological properties.
Imbalance in the barrier function and the lipid composition in and on the epidermis is associated with several different skin diseases such as atopic dermatitis (eczema), psoriasis, acne, ichthyosis (fish skin disease), rosacea and also common aged dry skin. For example, in atopic dermatitis, a significant reduction of certain ceramides and a higher concentration of certain unsaturated short-chain fatty acids has been observed, and in acne, a reduction in the chain length of the fatty acids in ceramides and an increase in the concentration of cholesterol and squalene have been observed.
Lipids that are applied to the skin through topical13 use, e.g. via skin care products, can help correct an imbalance in the lipid composition and alleviate some of the problems that some skin diseases and the skin can cause. Linoleic acid has been shown to strengthen the epidermal barrier, normalize epidermal water loss (TEWL14) and improve smoothness of the skin when used topically and orally. Some studies suggest that a high concentration of Oleic acid and at the same time a low concentration of Linoleic acid in skin care products can deteriorate the skin barrier and increase irritation in skin which is already impaired or not fully developed - for example skin with inflammation and the skin of infants. Skin with a normal barrier and without elevated levels of inflammation does not appear to be adversely affected by the fatty acid composition. Unsaturated fatty acids have been shown to have different properties in relation to the skin. For example, studies suggest that omega-9 fatty acids can induce faster wound healing, while omega-3 fatty acids can delay wound healing. Oils with a high level of Linoleic acid and saturated fatty acids have shown a positive effect on the skin barrier and clinical trials with Linoleic acid and polyunsaturated fatty acids derived from it have shown that they can reduce TEWL (improve the barrier) and have a soothing effect on the skin.
12A hydrocarbon is a molecule that consists exclusively of carbon and hydrogen.
13Topical use refers to using a product by applying it to the body's surfaces; thus, all cosmetics are used via topical administration.
14TEWL is th abbreviation for Trans Epidermal Water Loss. Measuring TEWL is often used to assess the skin's barrier function.
Oils and lipids in cosmetic
Oils, waxes and many other ingredients with lipids in them are important elements in many cosmetics, where they can be added for different purposes – it is typically one or more of the following:
- A technical purpose; e.g. dissolving hydrophobic active substances, dispersing pigments, ensuring that an emulsion does not separate, which amphiphilic emulsifiers usually provide and adjusting the viscosity of the product – wax can, for example, increase the viscosity.
- A sensory purpose; e.g. to give the right feeling on the skin/hair during and after application and for fragrant lipids to give fragrance to the product.
- A physical purpose; e.g. to soften and retain moisture in the skin, which most vegetable oils can contribute to.
- A biochemical purpose; for example to give the product an antimicrobial, anti-inflammatory or antioxidative effect, which certain lipids can give.
In addition to vegetable lipids "directly" from the plant, many derived lipids are also used; lipids which have been chemically changed in various ways to, for example, be more stable, more uniform and/or have other properties. An example of such a lipid is the widely used substance Caprylic/Capric Triglyceride. This is a triacylglyceride with primarily the fatty acids Caprylic acid and Capric acid in the three places on the glycerol molecule.
It is most often produced by hydrolyzing coconut oil, removing glycerol, separating the fatty acids to obtain a fraction with primarily Caprylic acid and Capric acid and finally re-esterifying the glycerol molecules with this fatty acid fraction.
Other examples are many emulsifiers, which often consist of a hydrophobic end in the form of a fatty acid, e.g. from palm oil, to which a hydrophilic end such as a chain of glycerol has been added. Lipids have many purposes, and you have both many lipids from nature and many different lipids have been developed to fulfill them.
Frequent vegetable oils
There are many vegetable oils that are extracted "directly" from nature, and many of the vegetable oils that are used in cosmetics are also used in food. In the following, some of the most common oils and other lipid-containing ingredients used in cosmetics are briefly described and, for a few, a little more description is given of what properties they have according to the scientific literature.15 , some of the most common oils and other lipid-containing ingredients used in cosmetics are briefly described and, for a few, a little more description is given of what properties they have according to the scientific literature.
- Adansonia Digitata Seed Oil – Baobab oil – contains approx. 35% Linoleic acid, 30% Palmitic acid and 25% Oleic acid. A small in vivo study has shown, for example, that it can reduce TEWL by forming a protective layer on top of the skin and thereby maintaining the moisture in the skin.
- Argania Spinosa Kernel Oil – Argan oil – is often used in hair products and contains approx. 80% unsaturated fatty acids, primarily divided into Oleic acid and Linoleic acid. Approx. 0.7-1% of the oil are unsaponifiable components. In vivo experiments have shown that this oil can improve skin elasticity and moisture by improving the skin barrier and retaining water in the skin.
- Borago Officinalis Seed Oil – Borage oil – is one of the oils that is described as a dry/light oil. It contains approx. 35% Linoleic acid, 20% Oleic acid, 10% Palmitic acid, 4% Stearic acid and as much as 20-30% γ-Linolenic acid, which is quite special for a vegetable oil. γ-Linolenic acid is often abbreviated GLA and is used, for example, in food supplements. In the body, γ-Linolenic acid is formed from the essential fatty acid Linoleic acid. The unsaponifiable components make up about 1-2% of the oil and are, for example, tocopherols and phenolic substances.
- Butyrospermum Parkii Butter – Shea butter - also called Karité butter and is a widely used and fairly soft and sensory desirable oil, with a melting point typically around 30-40 °C, which, like Mango butter, is advantageous for some products. The fatty acids in the triacylglycerides in shea butter are primarily Stearic acid and Oleic acid (about 40-45% of each) and to a lesser extent Palmitic acid, Linoleic acid and Arachidic acid. Compared to most other vegetable oils, Shea butter contains a very large proportion of unsaponifiable substances – around 7-10%. These are especially of the lipid category prenol lipids such as triterpene esters and unsaturated isoprenoids (e.g. tocopherol) and also sterols and phenols. One of the dominant triterpenes is the substance Lupeol, which has shown anti-inflammatory properties in in vitro studies. In in vivo experiments with animals, the substance has been shown to be able to alleviate various diseases such as arthritis. The content of triterpenes also means that shea butter has a weak UVB protective effect. In vivo studies have shown that shea butter can reduce some signs of aging and inhibit sun-induced aging in addition to having good emollient properties for both hair and skin.
- Cannabis Sativa Seed Oil – Hemp oil – is from the seeds of the hemp plant and usually contains less than 2% unsaponifiable components such as cannabinoids such as CBD16 and almost none of the psychoactive substance THC17. Among the unsaponifiable components are chlorophyll, which gives the oil its green color, tocopherol, carotenoids, phytosterols and terpenes. The fatty acid composition is as follows: Linoleic acid 55-65%, α-Linolenic acid 15-25%, Oleic acid 10-20%, Palmitic acid 6-8%, Stearic acid 2-3% and some varieties yield oil with up to 4% γ-Linolenic acid.
- Canola Oil /Brassica Campestris Seed Oil – Canola oil/rapeseed oil. Canola oil is from special rapeseed varieties that have been developed not to contain so much of the fatty acid Erucic acid, which has been shown to be harmful (by oral intake). The oil contains about 55% Oleic acid and about 25% Linoleic acid and about 5% saturated fatty acids - primarily Palmitic acid. 0.5-5% are unsaponifiable components.
- Carthamus Tinctorius Seed Oil – Thistle oil/safflower Oil - is one of the dry/light oils and in terms of the fatty acid composition, it mainly contains PUFA in the form of approx. 70% Linoleic acid. Tocopherols form a significant part of the unsaponifiable substances in this oil.
- Cera Alba – Beeswax – is a wax that can consist of more than 300 different substances, which can vary in relation to exactly which bee species it is from and what food they have eaten. This wax consists primarily of linear long-chain wax esters, some complex wax esters, free fatty acids (especially C24-32) and hydrocarbons with an odd number of C atoms. It may contain a small amount of pollen and propolis.
- Cocos Nucifera Oil/Cocos Nucifera Seed Butter – Coconut oil/coconut butter – is a vegetable butter as the melting point is usually around 25 °C. This product is usually completely white and contains over 99% triacylglycerides and only a small proportion of unsaponifiable components, which are primarily phytosterols and a little tocopherol (about 0.5%). The fatty acid composition of the triacylglycerides is as follows: approx. 50% Lauric acid, and approx. 5-10% of respectively Caprylic acid, Capric acid, Myristic acid, Palmitic acid and Oleic acid. It thus consists of more than 90% saturated fatty acids and mostly those with medium chain length, which is special for coconut oil. Something else special is that it contains a lot of mono-laurin - i.e. triacylglyceride, where all three fatty acids are lauric acid. Coconut oil in virgin quality has been shown in studies to promote wound healing and the oil has also shown antimicrobial, antifungal and antiviral activity. In studies with atopic dermatitis patients, coconut oil has shown a significant inhibition of the bacterium Staphylococcus aureus and improvement of the skin. Due to the high content of saturated fatty acids, coconut oil has been considered unhealthy to eat, but studies have gradually shown more health-promoting properties when consuming coconut oil.
- Elaeis Guineensis Oil and Elaeis Guineensis Kernel Oil – Palm Oil and Palm Kernel Oil – is sometimes used in hydrogenated form and is often used to make other substances such as surfactants.
- Helianthus Annuus Seed Oil – Sunflower oil - mainly contains Linoleic acid (approx. 60%) and Oleic acid (approx. 30%), but can also be obtained with, for example, a particularly high content of Oleic acid. This oil is one of the widely used oils in cosmetics and food.
- Lanolin – Lanolin – is fat and wax from sheep's wool, which the sheep secretes from sebaceous glands in the skin. It is thus one of the relatively few animal oils used in cosmetics. Lanolin can be fractionated into, for example, a wax part (Lanolin Cera) and an oil part (Lanolin Oil) and these can be further fractionated and, like other lipids, chemically modified. Lanolin is a complex mixture of primarily long-chain wax esters, sterol esters, triterpenes and fatty alcohols and fatty acids - it does not contain triacylglycerides like most vegetable oils. Lanolin has a good water absorption capacity.
- Limnanthes Alba Seed Oil – Meadowfoam oil – is rich in MUFA and especially long-chain fatty acids ≥ C20, which is special for this oil, which together with the tocopherol content gives the oil a high oxidative stability and special sensory properties.
- Macadamia Integrifolia Seed Oil/Macadamia Ternifolia Seed Oil – Macadamia oil – contains a lot of MUFA as it contains 50-65% Oleic acid and 10-20% Palmitoleic acid, which is a high level compared to many other vegetable oils. In addition, it contains 7-12% Palmitic acid, 2-9% Linoleic acid, 0-15% α-Linolenic acid and a relatively high level of Tocopherol and Squalene and some phytosterols and polyphenols. The oil is reasonably stable against oxidation and also has good sensorial properties in relation to cosmetics.
- Mangifera Indica Seed Butter/Mangifera Indica Seed Oil – Mango butter – is one of the slightly newer vegetable solid oils with a melting point around 35 °C, so it melts on contact with the skin, which is desirable for some cosmetic products. The fatty acid composition is as follows: 38-45% Oleic acid, 35-45 Stearic acid, 7-8% Palmitic acid, 4-6% Linoleic acid and approx. 2% Arachidic acid. The oil contains 0.7-2.4% unsaponifiable components, and these are primarily tocohperol, phytosterol (especially b-sitosterol and stigmasterol and campesterol) and triterpenes (e.g. Lupeol). This oil has a high oxidative stability and emollient properties.
- Olea Europaea Fruit Oil – Olive oil – is one of the sensory fatter oils and contains especially triacylglycerides with Oleic acid (55-80%) and less Linoleic acid and Palmitic acid. Over 200 different lipids have been found in olive oil – for example, the unsaponifiable part contains carotenoids, phenols and a reasonably high level of squalene. Part of the Squalane used in cosmetics is extracted from the unsaponifiable part of olive oil.
- Persea Gratissima Oil – Avocado oil – contains 47-60% Oleic acid and the concentration of unsaponifiable components can vary greatly (0.4-12.2%), which i.a. is reflected in the color which can be from light yellow to dark green. In vivo experiments on rats have shown that topical use can increase collagen synthesis and reduce the number of inflammatory cells in the wound healing process.
- Prunus Amygdalus Dulcis Oil – Almond oil – is from the sweet almond and not bitter almond. The oil contains 60-85% Oleic acid, 20-30% Linoleic acid, 3-9% Palmitic acid and also tocopherol.
- Ricinus Communis Seed Oil – Castor oil - is an example of one of the fatty oils that sits on top of the skin, which can make it suitable for massage oil. Something very special about this oil is that it contains 80-90% of the fatty acid Ricinoleic acid (C18:1, omega 9, which has a hydroxyl group (OH group) on C12) – such a high level of this fatty acid is not in other vegetable oils. It is one of the oils that is occasionally used in its hydrogenated form, where the INCI name is Hydrogenated Castor Oil. The color of the oil can vary from almost colorless to reddish brown.
- Rosa Rubiginosa Seed Oil/Rosa Canina Seed Oil – Rosehip seed oil – contains 35-55% Linoleic acid, 15-25% α-Linolenic acid and 15-23% Oleic acid as well as unsaponifiable components such as carotenoids and tocopherol. Something special about this oil is that it can also contain the substance all-trans-retinoic acid – a retinoid (vitamin A), which is not allowed to be added to cosmetics in the EU. This substance is used, for example, in anti-acne medicines, where it can be a skin irritant. However, rosehip seed oil is not irritating to the skin and due to the natural content of retinoids, the oil is believed to be able to provide some of the positive effects that vitamin A is known to have on the skin.
- Simmondsia Chinensis Seed Oil – Jojoba oil – is called an oil but is actually a liquid wax, which is very special. This liquid wax makes up as much as 50% of the seed's weight and consists of approx. 98% wax - primarily wax esters and small proportions of free fatty acids, fatty alcohols, hydrocarbons, triacylglycerides, sterols and tocopherols. The wax esters consist of long-chain, unbranched fatty acids and long, unbranched fatty alcohols, where both chains are typically C20-22 and some are unsaturated omega-9 chains. Jojoba oil has a very high oxidative stability, and the special chemical composition is comparable to the wax part of the skin's natural sebum. It has been shown to have a positive softening effect on the skin and can increase the skin's elasticity. Studies also suggest that jojoba oil can help with/in wound healing and that the oil has antioxidant, antiviral, antimicrobial and anti-inflammatory effects. In several trials with medicines for topical use, jojoba oil has been shown to be a good delivery system and thus be able to promote the active substance's path to the site of action in the skin and thus the therapeutic effect.
- Squalane and Squalene – Squalane and Squalene – are lipids belonging to the category of Prenol lipids and in this category the subcategory of C30 isoprenoids (triterpenes). The difference between Squalene and Squalane is that the former contains six double bonds, and these have been removed in Squalane, which makes Squalane more stable. Squalene is found in nature - and as mentioned also in the skin of humans - and was originally mainly extracted from shark liver oil, which is not considered ethically sound today. Today, for example, it can be extracted from olive oil, and it can also be produced by fermenting sugar with subsequent chemical processes. Squalene is then most often hydrogenated to the more stable Squalane when used in cosmetics. In its pure form, Squalane is a clear, odorless and dry oil which is easy to spread and provides a non-greasy and silky feel on the skin. In vivo studies have shown that the concentration of Squalene in the skin decreases with age and that Squalane can reduce wrinkles and improve skin elasticity and can make hair easier to comb.
- Triticum Vulgare Germ Oil – Wheat germ oil – is rich in PUFA as the fatty acid composition of the triacylglycerides is as follows: 45-60% Linoleic acid, 10-20% Palmitic acid, 14-25% Oleic acid, 4-10% α-Linolenic acid and 2% Stearic acid. The unsaponifiable parts make up about 4% of the oil and tocopherols – primarily α-tocopherol – make up to 0.3% of the oil, which is one of the highest concentrations found in vegetable oils. The other unsaponifiable parts are e.g. carotenoids, sterols, squalene, phenols and ceramides, which is interesting in relation to cosmetic use.
- Vitis Vinifera Seed Oil – Grape seed oil – contains like safflower oil, approx. 70% Linoleic acid and has a relatively high content of tocopherols, which contributes to the oxidative stability of the oil.
15In the list, the primary INCI name is listed first and then the common English name(s). The stated percentages for fatty acid composition and unsaponifiable parts may vary depending on, for example, the manufacturing method and subsequent refining processes.
16CBD is the abbreviation for cannabidiol, which is one of over a hundred identified cannabinoids found in the hemp plant.
17THC is the abbreviation for tetrahydrocannabinol – the most psychoactive substance in the hemp plant.
Sources
- Ahmad, A.; & Ahsan, H. Lipid-based formulations in cosmeceuticals and biopharmaceuticals. Biomedical Dermatology. 2020; 4, 12.
- Alvarez, A.M., & Rodríguez, M. Lipids in pharmaceutical and cosmetic preparations. Grasas Y Aceites. 2000; 51, 74-96.
- Amyris, Neossance Squalane presentation April 2015 & https://aprinnova.com/neossance-squalane/ Lokaliseret 14. marts 2023.
- Andersson, A.-C. Shea Butter Extract for Bioactive Skin Care: https://www.cosmeticsandtoiletries.com/research/literature-data/article/21835355/shea-butter-extract-for-bioactive-skin-care. 2015. Lokaliseret 2. marts 2023.
- Archambault, J.-C. Vegetable fats in cosmeticology. Revista voliviana de QuímiCa. 2021 38. 80-94.
- Asadi-Samani, M.; Bahmani, M.; & Rafieian-Kopaei, M. The chemical composition, botanical characteristic and biological activities of Borago officinalis: a review. Asian Pacific journal of tropical medicine. 2014; 7S1, S22–S28.
- Blaak, J; & Staib, P. An updated review on efficacy and benefits of sweet almond, evening primrose and jojoba oils in skin care applications. International Journal of Cosmetic Science. 2022; 44: 1– 9.
- Christie, W. The LipidWeb: https://www.lipidmaps.org/resources/lipidweb/lipidweb_html/index.html. Lokaliseret 3. marts 2023.
- Concha, J.; Soto, C.; Chamy, R.; & Zuñiga, M. Effect of rosehip extraction process on oil and defatted meal physicochemical properties. Journal of Oil & Fat Industries. 2006; 83. 771-775.
- De Luca, M.; Pappalardo, I.; Limongi, A.R.; Viviano, E.; Radice, R.P.; Todisco, S.; Martelli, G.; Infantino, V.; & Vassallo, A. Lipids from Microalgae for Cosmetic Applications. Cosmetics. 2021; 8, 52.
- Fahy, E.; Cotter, D.; Sud, M.; & Subramaniam, S. Lipid classification, structures and tools. Biochimica et biophysica acta. 2011; 1811(11), 637–647.
- Ferreira, M. S.; Magalhães, M. C.; Oliveira, R.; Sousa-Lobo, J. M.; & Almeida, I. F. Trends in the Use of Botanicals in Anti-Aging Cosmetics. Molecules (Basel, Switzerland). 2021; 26(12), 3584.
- Franco, A.; Salvia, R.; Scieuzo, C.; Schmitt, E.; Russo, A.; & Falabella, P. Lipids from Insects in Cosmetics and for Personal Care Products. Insects. 2022; 13, 41.
- Gad, H. A.; Roberts, A.; Hamzi, S. H.; Gad, H. A.; Touiss, I.; Altyar, A. E.; Kensara, O. A.; & Ashour, M. L. Jojoba Oil: An Updated Comprehensive Review on Chemistry, Pharmaceutical Uses, and Toxicity. Polymers. 2021; 13(11), 1711.
- Ghafoor, K.; Özcan, M.; AL Juhaimi, F.; Babiker, E.; Sarker, Z.; & Mohamed, I.; & Ahmed, M. Nutritional Composition, Extraction and Utilization of Wheat Germ Oil: A Review. European Journal of Lipid Science and Technology. 2016; 119.
- Huang, Z. R.; Lin, Y. K.; & Fang, J. Y. Biological and pharmacological activities of squalene and related compounds: potential uses in cosmetic dermatology. Molecules (Basel, Switzerland). 2009; 14(1), 540–554.
- Jungersted, J. M.; Hellgren, L. I.; Jemec, G. B.; & Agner, T. Lipids and skin barrier function--a clinical perspective. Contact Dermatitis. 2008; 58(5), 255–262.
- Kaseke, T.; Fawole, O.A.; & Opara, U.L. Chemistry and Functionality of Cold-Pressed Macadamia Nut Oil. Processes. 2022; 10, 56.
- Kendall, A. C.; & Nicolaou, A. Topical application of lipids to correct abnormalities in the epidermal lipid barrier. The British journal of dermatology. 2022; 186(5), 764–765.
- Knox, S.;& O'Boyle, N. M. Skin lipids in health and disease: A review. Chemistry and physics of lipids. 2021; 236, 105055.
- Komane, B. M.; Vermaak, I.; Kamatou, G. P. P.; Summers, B.; & Viljoen, A. M. Beauty in Baobab: a pilot study of the safety and efficacy of Adansonia digitata seed oil. Revista Brasileira de Farmacognosia. 2017; Vol 27(1), 1-8.
- Lin, T. K.; Zhong, L.; & Santiago, J. L. Anti-Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. International journal of molecular sciences. 2017; 19(1), 70.
- Malachi, O. Effects of Topical and Dietary Use of Shea Butter on Animals. American Journal of Life Sciences. 2014; 2. 303-307.
- Mármol, I.; Sánchez-de-Diego, C.; Jiménez-Moreno, N.; Ancín-Azpilicueta, C.; & Rodríguez-Yoldi, M. J. Therapeutic Applications of Rose Hips from Different Rosa Species. International journal of molecular sciences. 2017; 18(6), 1137.
- Mnekin, L.; & Ripoll, L. Topical Use of Cannabis sativa L. Biochemicals. Cosmetics. 2021, 8, 85.
- Natesan, V.; & Kim, S. J. Lipid Metabolism, Disorders and Therapeutic Drugs - Review. Biomolecules & therapeutics. 2021; 29(6), 596–604.
- Nutritional Composition, Extraction and Utilization of Wheat
- O'Lenick, A. Oils and Butters for Cosmetic Applications. Personal Care. 2016, 32.
- Pal, P.K.; Rathva, D.; Parmar, D.; Patel, J.; Upadhyay, S.; & Umesh, U. A Review on Coconut oil: An Essential Oil for All. Research & Review: Journal of Pharmacognosy and Phytochemistry. 2020; 9(1), 27-32.
- Poljšak, N.; & Kočevar Glavač, N. Vegetable Butters and Oils as Therapeutically and Cosmetically Active Ingredients for Dermal Use: A Review of Clinical Studies. Frontiers in pharmacology. 2022; 13, 868461.
- PubChem Sketcher V2.4. Lokaliseret 24. marts 2023: https://pubchem.ncbi.nlm.nih.gov
- Shukla, V.; & Bhattacharya, K. Mango Butter in Cosmetic Formulations. Cosmetics & Toiletries. 2002, 117 (6), 65.
- Thompson, T. E. Lipid. Encyclopedia Britannica. https://www.britannica.com/science/lipid (sidst opdateret 4. marts 2023). Lokaliseret 14. marts 2023.
- Website Lipid Maps® Lokaliseret 18. marts 2023: https://www.lipidmaps.org/
- Wikipedia webside:
Vegetable oil: https://en.wikipedia.org/wiki/Vegetable_oil;
Triglyceride: https://en.wikipedia.org/wiki/Triglyceride. Lokaliseret 1. marts 2023.
