Antioxidants and Oxidants
Antioxidants and Oxidants
Antioxidants
Antioxidants are a large group of very diverse substances that are found everywhere. Every cell in an environment with oxygen – whether it is in a human, a fish, a plant, or is a single-celled organism such as a bacterium – contains antioxidants. Paradoxically, oxygen is vital for most cells on earth, and at the same time oxygen is a very reactive substance which can form oxidants substances that easily can oxidize other substances nearby. Oxidation can damage the function of a substance. This paradox is called the paradox of aerobic1 life. Therefore, in each cell, there is an antioxidant network of substances that can prevent, inhibit or repair the oxidative damage that oxygen can cause to cellular components.
Antioxidants are defined by their ability (in relatively low concentrations) to prevent or reduce oxidation of other molecules caused by oxidants. This can occur by preventing the formation of oxidants, or by neutralizing the oxidants and thus preventing them from oxidizing other molecules. A slightly broader definition of antioxidants also includes substances which remedy the damage caused by oxidants - e.g. DNA repair enzymes - and some also include other substances which in a more indirect way help in the antioxidant defense - e.g. metals which are necessary to some antioxidants work, substances that bind certain metals that can otherwise contribute to the formation of oxidants and substances that can increase the activity of antioxidants. Here, the focus will be on the substances that fall under the first definition.
1Aerobic refers to biological processes that require oxygen. Anaerobic refers to biological processes that do not require oxygen.
Products with Antioxidants
The balance between oxidants and antioxidants
The balance between oxidants and antioxidants - the so-called redox balance - is important for the cell's functions. When the balance tips in favor of the oxidants, so-called oxidative stress occurs, which has been linked to many different disorders. The balance can also shift in the other direction, but this has not been studied much. As in most other contexts, when talking about life, it is about finding and maintaining a balance, because oxidants are not only problematic, as was first believed, but are also necessary for a number of vital processes; and antioxidants are not only good but can also have negative effects in too high a concentration.
Many studies have been carried out with antioxidants to, for example, find out whether they can prevent or treat diseases. Clinical studies with humans have shown mixed results, so it is still debated whether antioxidants in the form of food supplements can promote health. There is no doubt that foods containing antioxidants promote health, but it does not seem to be the antioxidants alone that provide the positive effects.
The use of Antioxidants
Antioxidants are found in and added to foods, for example, and many antioxidants are also used in cosmetics, where they are believed to be able to remedy the aging that oxidants are believed to be able to cause. PUCA PURE & CARE uses many different antioxidants in its products, many of which are in the form of plant extracts from e.g. the tea bush Camellia Sinensis, the witch hazel plant Hamamelis and Aloe Vera and others are in the form of the isolated substances such as Coenzyme Q10 (Ubiquinone) and derivatives of Vitamin C and E.
Oxidants and antioxidants - chemical introduction
Oxidants is a broad term for substances which can oxidize other substances – they can also be called pro-oxidants. In a biological context, free radicals, ROS and the slightly less known RNS are often mentioned. ROS is the abbreviation for Reactive Oxygen Species; reactive oxygen compounds and RNS is the abbreviation for Reactive Nitrogen Species; reactive nitrogen compounds (of which the biologically relevant ones also contain oxygen). These three designations partially overlap, so, for example, some free radicals are also ROS and RNS, but there are also free radicals that do not go under the ROS/RNS designations and there are ROS and RNS that are not radicals. The word "ROS" is often used as a collective term for the biologically relevant oxidants.
How do the Oxidants work?
To explain the effect of oxidants, one must look a little more closely at the structure of atoms and redox reactions: An atom generally contains a number of protons and neutrons, which make up the nucleus, and around the nucleus there is a number of electrons, which move in certain orbits, which are ordered in shells, with different distances to the core. Normally, the electrons are found in pairs, but in free radicals there are one or more unpaired electrons in the outermost shell, which makes the radical unstable and reactive. In order to achieve stability, an electron must normally be found for the unpaired electron – and the radical typically finds this in a neighboring molecule or atom. This reaction where an electron is transferred from one substance to another is called a redox reaction. See figure 1. "Redox" is a contraction of the words "reduction" and "oxidation". The radical, which takes the electron, is reduced, while the neighboring molecule or atom, which gives up an electron, is oxidized – and at the same time, if the neighboring molecule or atom was not already a radical, it becomes a radical itself. Thus, this new radical can continue the reaction and take an electron from another molecule, etc. This is called a radical chain reaction, and this will continue until there are two radicals involved in the redox reaction or it is a type of antioxidant that can handle losing an electron without becoming reactive, which is part of the redox reaction. In a similar way, non-radical ROS molecules can participate in redox reactions or be converted into radicals, which then can participate in redox reactions.
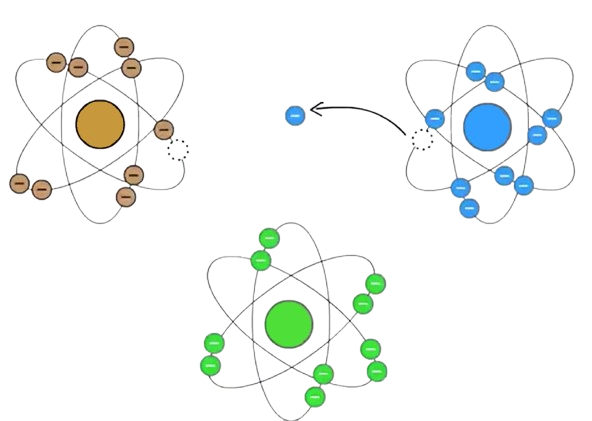
Figure 1: Illustration of a redox reaction between a radical (illustrated by a brown atom), which has an unpaired electron, and a substance (the blue atom), which can, for example, be a molecule with antioxidant properties, which can deal with losing an electron without becoming a reactive radical. The bottom figure (the green atom) represents a neighboring substance which cannot give up an electron without becoming an unstable and reactive radical.
Antioxidants are both molecules that can prevent a reactive radical from being formed from e.g. a ROS and molecules that, as described above, can participate in redox reactions with ROS including radicals – these are often also called free-radical scavengers – and these become oxidized in the process and act as so-called reducing substances (they reduce the radical). In that process, the antioxidant can risk becoming a pro-oxidant and thus be able to oxidize other substances instead of reducing them. In the human body you have a complex network of antioxidants that help each other dealing with ROS and thus maintain the redox balance. In the following two sections, ROS and antioxidants will be described in more detail.
ROS
- Free radicals
Reactive oxygen compounds, ROS, are generally relatively small molecules that want to take an electron and thus oxidize a neighboring molecule or atom, which, can lead to a radical chain reaction. The molecules and atoms involved in such a reaction may risk being changed and thereby lose their function. The substances that ROS most often attack are unsaturated lipids2 (fats), proteins and nucleic acids (DNA and RNA). Lipids and proteins make up the majority of each cell's membrane and therefore oxidation of these can have a major impact on the cell's function. Lipids also make up a large part of, for example, the skin's sebum, and oxidation of this can affect the skin and is, for example, part of the development of acne. Proteins perform a very wide range of functions in the body. All the body's enzymes, which catalyze an incredible number of processes and, for example, the immune system's antibodies, are proteins. There are structural molecules which are proteins and hormonal proteins. Thus, oxidation of proteins can have very different effects in the body. Oxidation of nucleic acids can cause mutations, which - if not corrected - can contribute to the development of cancer, for example. Oxidation and other forms of damage to molecules happen all the time in the body, which the system handles by repairing the damage or removing and replacing the molecules that are damaged. It is naturally resource-intensive and a balance must be maintained so that many damaged molecules do not accumulate. With oxidative stress, the balance is tipped so that there are more ROS than the antioxidative defense can handle – which results in more damaged molecules than the body's repair and clean-up system can keep up with.
What are the functions of free radicals?
ROS are often described as something that must be fought and removed, but it is important to point out that ROS are also necessary for the body, as they are part of various vital processes. For example, the immune system uses ROS to fight micro-organisms and ROS are also cell signaling substances in, for example, muscle contraction and in regulating blood pressure. Therefore, the body need a balanced level of ROS3.
ROS are formed naturally in the body (endogenous ROS) in connection with, for example, metabolism, where oxygen must be used - especially in the cells' mitochondria, where the body's energy "currency", ATP4, is formed via the electron transport chain. In this process, electrons are moved between different molecules and in this connection, for example, the ROS molecule superoxide anion radical is formed as a by-product if an electron escapes from the electron transport chain and reacts with oxygen (O2). ATP and ROS are produced all the time in the body – and more is formed in connection with e.g. infections, where the immune system also produces ROS and during exercise, when the cells consume more ATP. The formation of ROS can also be induced by external influences (exogenous ROS) such as pollution and the sun's rays.
The following list contains some of the biologically most important ROS:
Superoxide anion (02-·) is a ROS and also a radical. It is formed, for example, in the electron transport chain in the cells' mitochondria and by the sun's UV rays. The superoxide anion radical is the precursor for most other ROS.
Hydrogen peroxide (H2O2) is a more stable ROS. They can be formed from superoxide anion via the antioxidant enzymes superoxide dismutase, as part of the fight against ROS.
Hydroxyl radical (·OH) is a highly reactive ROS and also a free radical which reacts very quickly and non-specifically with most molecules. Hydroxyl radicals are formed, for example, from hydrogen peroxide in a metal-catalyzed redox reaction5, where hydrogen peroxide is converted into hydroxyl radical and hydroxide ion (OH-).
Hypochlorous acid (HClO) is a ROS, which are, for example, formed in immune cells to fight microorganisms.
Nitric oxide (NO·) is a radical RNS which is both water- and fat-soluble and can therefore easily get around in the body. It is an important signaling molecule in the body, which controls various physiological functions such as blood pressure and relaxation of certain muscles and it also plays an important function in the immune system in connection with inflammation. The production of Nitric oxide is normally tightly regulated.
Peroxynitrite (ONOO-) is a very reactive RNS anion, which can for example be formed from hydrogen peroxide and nitrite and by superoxide anion reacting with nitric oxide.
Peroxyl radical (ROO·) is a term for a type of the radicals that e.g. lipids can be converted into when they react with a ROS and are thus oxidized to a radical molecule that can participate in a radical chain reaction and thus oxidize other molecules.
2Unsaturated lipids are fats that contain one or more double bonds from which ROS can take an electron. Lipid peroxidation is called this process where the radical chain reaction takes place with lipids, which breaks them down.
3You can read a very thorough historical and technical review of the development of knowledge within oxidants in the following article: Evolution of the Knowledge of Free Radicals and Other Oxidants. Written by Di Meo, S. & Venditti, P. in the journal 'Oxidative medicine and cellular longevity'. 2020, Artikel ID 9829176.
4ATP is the abbreviation for Adenosine TriPhosphate.
5The metal in these reactions is typically iron and copper. Two well-known reactions form hydroxyl radicals – these are called the Fenton and Harber-Weiss reactions.
The Antioxidants
- The three lines of defense
The antioxidants are the body's response to the oxidants, ROS – the balance between them is important for the functions of each cell. This response can be divided into three "defense" lines: First are the antioxidants that inhibit the formation of oxidants - these are, for example, enzymatic antioxidants. The next line is the antioxidants which inhibit the oxidants from reacting with other molecules and thus break the radical chain reaction – these are, for example, smaller molecules that can give up an electron. The third line is the substances (which are not always classified as antioxidants) which have a more indirect effect by repairing the damage and promoting the formation and/or activity of antioxidants. Several different interactions take place between the different antioxidants. For example, some antioxidants help other antioxidants to be regenerated to their reduced form, so that they are again ready to donate an electron to an oxidant6.
The different properties of the antioxidants
The antioxidative properties of antioxidants can be measured in many different ways7 And each measurement method has its advantages and disadvantages. Antioxidants have different affinity for the different oxidants, so one antioxidant cannot handle all oxidants. This is often reflected in many studies as it is concluded that antioxidants generally work better in combination. You can group antioxidants in many ways. There are, for example, the water-soluble and the fat-soluble - this is decisive for where in the body they work, as the water-soluble will generally be in water-containing areas such as inside the cell, while the fat-soluble will generally be in, for example, the cell membrane. You can also divide them into the antioxidants that the body itself produces and the antioxidants that you have to consume in order to benefit from them. Antioxidants can also be divided into those that are enzymes and those that are not - and those that are chemically produced and invented by humans (synthetic) and those that are naturally produced in nature (natural). The vast majority of antioxidants are natural, but there are also some that have been developed by humans and are, for example, used in food and cosmetics.
6Read about the interaction between vitamin E and C in the descriptions of these ingredients.
7You can read much more about this in the following article: Rasheed, A., & Azeez, R. F. A. A Review on Natural Antioxidants. Chapter 5 in C. Mordeniz, Traditional and Complementary Medicine. IntechOpen. 2019.
The following list contains examples of important antioxidants and groups of antioxidants:
Superoxide dismutase (SOD)
Is a group of enzymes that catalyze the conversion of superoxide anion to oxygen and hydrogen peroxide, which can then be handled by other enzymes. Superoxide dismutase is found in almost all aerobic organisms and, for example, in food such as cabbage and wheat. The human body itself is capable of producing these enzymes, which are distributed throughout the body - also in the skin, where they also play an important role in the formation of fibroblasts. To function, the various sod enzymes must use specific metal ions as cofactors. The metals used are copper, zinc, iron, manganese or nickel. Therefore, superoxide dismutases are so-called metalloenzymes.
Catalase (CAT)
Is another group of enzymes that the human body can produce itself and which are also metalloenzymes because they need manganese or iron as cofactors. Catalase takes over the further processing of hydrogen peroxide from Superoxide dismutase and converts it into water and oxygen. This process happens very quickly and efficiently so that a catalase enzyme can convert approximately 6 million molecules of hydrogen peroxide into oxygen and water every minute – this is one of the highest turnover rates among enzymes.
Peroxiredoxins
Are a group of peroxidase enzymes which can also catalyze the breakdown of hydrogen peroxide and also peroxynitrite. These enzymes do not depend on a metal-ion cofactor and they are produced in the human body.
Glutathione peroxidase (GPx)
Is another group of enzymes that the body produces itself and that use selenium as a cofactor. Like catalase, glutathione peroxidase has a high affinity for hydrogen peroxide, which it can convert to water. Furthermore, they can also convert lipid peroxides into lipid alcohols. Glutathione peroxidase is part of the glutathione system, which consists of a collaboration with the tri-peptide glutathione, which acts as a coenzyme (auxiliary substance) for glutathione peroxidase and the enzyme group glutathione reductase, which takes care of reducing glutathione to its reduced active form. This system is found in humans, animals, plants and microorganisms.
Thioredoxin reductase
Is another enzyme group which, together with the antioxidant protein thioredoxin (Trx), forms part of the thioredoxin system, which constitutes a central antioxidant system in many organisms. This system can reduce disulfide bonds in e.g. oxidized proteins. The thioredoxin reductase enzyme uses NADPH8 as an electron donor to catalyze the reduction and thus the activation of thioredoxin.
Coenzyme Q10
Which in reduced, partially oxidized and oxidized form is also called ubiquinol, semi-quinone and- or ubiquinone. This coenzyme group is produced in both humans, animals and most bacteria and plays a very important role in the electron transport chain, which ensures the production of the body's energy currency, ATP. Compared to proteins, it is a very small and fat-soluble molecule. It acts as an antioxidant as it can give up two electrons (therefore it exists in three redox stages).
Glutathione (GSH)
Is a small water-soluble and sulfur-containing tri-peptide (consists of three amino acids) which can be reversibly oxidized and reduced and thus act as a redox-active antioxidant. This substance is probably one of the body's most important antioxidants, which is produced in most aerobic organisms. It is in itself an antioxidant and, as mentioned, it is also part of the glutathione system, where it acts as a coenzyme for glutathione peroxidase, which uses it to reduce and thus neutralize oxidants. In that reaction, glutathione is oxidized and forms a bond with another oxidized glutathione via a disulfide bond, which can then be reduced back to the active glutathione form – this reaction is catalyzed by the enzyme glutathione reductase, which uses the coenzyme NADPH to donate the electrons.
Uric Acid
Is a small water-soluble compound that is produced in the body and occurs in very high concentration in the blood, where it acts as an antioxidant against hydroxyl radicals, peroxynitrite and hypochlorous acid, for example.
Melatonin
Is a small natural hormone that, for example, controls the circadian rhythm and which the body produces. It works both as a direct antioxidant by being able to give up an electron (but, unlike many other antioxidants, it cannot be re-reduced and is thereby a terminal antioxidant) and as an indirect antioxidant as it can, for example, stimulate the activity of antioxidant enzymes.
Melanin
Is a group of compounds that give the color to the skin and protect the skin against the sun's rays and can thereby reduce the formation of oxidants in the skin. Melanin is thus not a classic antioxidant.
Vitamin C, E and A
Are groups of well-known antioxidants9. Vitamins are generally not produced in the body, which is why these must be consumed with food. vitamin C is a water-soluble redox-active antioxidant which, for example, can reduce hydrogen peroxide and also cooperates with, for example, vitamin E and glutathione to maintain the redox balance. It has been observed that there is generally a higher concentration of vitamin C in the epidermis compared to the dermis. Vitamin A and E are fat-soluble and especially vitamin E is known to protect the fats in cell membranes by inhibiting lipid peroxidation. vitamin E can be re-reduced to the active form by reaction with, for example, vitamin E, coenzyme Q10 and beta-carotene.
Carotenoids
Are a large group of fat-soluble, yellow-orange-red substances, which are found, for example, in many vegetables. There are over 700 naturally occurring carotenoids – examples include lycopene, lutein, zeaxanthin and probably the most studied carotenoid, beta-carotene. Six of these make up over 95% of the carotenoids that are found in the blood of humans10 , and these are also found in the skin. Animals do not produce carotenoids themselves. Around 16% of the ingested beta-carotene is converted in the human body into retinol – i.e. beta-carotene is a precursor to retinol (a vitamin A derivative). Together with vitamin E, they help to inhibit lipid peroxidation - and in addition, beta-carotene also has other functions in, for example, the immune system.
Phenols
Phenols are a very large group of very different substances which are mainly produced in plants - some of which have antioxidant properties. Phenols can generally be grouped into four subgroups:
- Phenolic acids; e.g. Caffeic Acid and Salicylic Acid
- Phenolic monoterpenes; e.g. Eugenol and Menthol – these are typically volatile substances in e.g. essential oils.
- Phenolic diterpenes; e.g. Canosol
Polyphenoles, which also can be grouped into four sub-groups:
- Flavonoids, which are a group of over 5000 substances; e.g. Quercetin, Curcumin, and Catechin.
Some of the flavonoids have been shown to be antioxidants and some are also anti-inflammatory, anti-vital, anti-carcinogenic and chelating agents (bind metals). - Tannins, which are found, for example, in wine and tea.
- Lignans, which are found, for example, in seeds and whole grains
- Stilbenes; e.g. Resveratrol, which is a known antioxidant in e.g. grapes.
Synthetically produced antioxidants
Synthetically produced man-made antioxidants such as Butylated Hydroxyanisole (BHA) and Butylated Hydroxytoluene (BHT). These have been and are still used in e.g. food, but are gradually being replaced by natural antioxidants, as some studies indicate that they can be harmful to humans.
Chelating agent – compounds which can bind metals – are not antioxidants, but can work in the antioxidative system by binding metals such as iron and copper, which can otherwise catalyze the formation of ROS. Examples of metal binders are Citric Acid, EDTA and Phytic Acid.
Selenium and zinc are sometimes mentioned as mineral antioxidants. They are not antioxidants in the classical sense, but contribute by being cofactors for antioxidant enzymes (e.g. glutathione reductase and superoxide dismutase).
8NADPH is the reduced form of the substance Nicotinamide Adenine Dinucleotide Phosphate, which is a coenzyme that is involved in over 40 reduction-reaction processes in the body.
9You can read more about these vitamins in the descriptions of these on this website.
10These six carotenoids are beta-carotene, beta-cryptoxanthin, alpha-carotene, lycopene, lutein and zeaxanthin.
Oxidative stress, health and aging
- Imbalance between oxidants and antioxidants
Oxidative stress is believed to be an important factor in many different diseases and conditions, but it is not clear in all cases whether the oxidative stress is a factor in the development of the condition or occurs as a consequence of the condition. Examples of diseases and conditions where oxidative stress is thought to play a role are, for example, alzheimer's, parkinson's, rheumatoid arthritis, diabetes, asthma, some forms of cancer and eye diseases and various inflammatory diseases and also in aging. Since oxidative stress is the imbalance between oxidants and antioxidants, research is conducted, and speculation is made as to whether antioxidants can remedy and/or prevent the conditions. Many in vitro studies suggest it, but it has not been proven clearly in the clinical in vivo studies done with humans. They have shown mixed results, which is why it is still debated whether antioxidants in the form of dietary supplements can promote health. In particular, clinical studies have been carried out with vitamin antioxidants as dietary supplements and they do not show that they can, provide a significant reduction in risk or the development of, for example, cardiovascular diseases. Some studies suggest, on the contrary, that dietary supplements with some antioxidants (vitamin a and beta-carotene) can cause a small increase in mortality among elderly and vulnerable groups and that beta-carotene can increase the incidence of lung cancer among smokers. Overall, studies suggest that a healthy lifestyle with adequate nutrition is important for health and is associated with the prevention of certain diseases, but it is not only because of the content of certain antioxidants it is more complex.11.
Oxidative stress and aging in the skin
In relation to the aging of the body, including the skin, there is the so-called "free radical theory of aging" - a theory that has been developed over time as new knowledge has emerged. The theory suggests that oxidants and the damage they cause is a decisive factor in aging. It is still a theory, but many studies support it and in general researchers agree that cell damage caused by oxidants and oxidative stress contributes to aging. It is also consistent with studies indicating that the activity of antioxidants that are formed in the body and thus the endogenous antioxidative defense is reduced with age and at the same time more oxidative damage in the body emerge. Therefore, it is plausible that antioxidants can delay aging.
Aging of the skin occurs as a result of several complex mechanisms, which are often divided into intrinsic and extrinsic mechanisms of aging. The intrinsic or chronological aging is the inevitable aging that occurs as a result of internal physiological factors such as genes and hormones and is believed to make up around 5% of aging in the form of e.g. thinner and drier skin with fine wrinkles. Extrinsic aging occurs as a result of various environmental influences such as pollution, nutrition and sunlight, of which the sun's UV rays are believed to make up approximately 80% of extrinsic aging. Extrinsic aging is believed to be the cause of coarser wrinkles, reduction of skin elasticity, changes in skin texture and skin tone and possibly the increase in the thickness of the epidermis (the outermost skin layer).
11You can read more about antioxidants in relation to dietary supplements, foods and diseases here: https://www.hsph.harvard.edu/nutritionsource/antioxidants/
12Topical use means that a product is used by placing it on the body's surfaces; thus, all cosmetics are used via topical administration.
13UV filters in sunscreens can also contribute to the inhibition of the signs of skin aging.
14You can read more about the structure of the skin in the description of glycerin on this website.
Contributing factors to the aging of the skin
Oxidative stress is a contributing process in aging, but does not stand alone, in both the intrinsic and extrinsic aging mechanisms. Other processes include, for example, the phenomenon of "inflamm-aging", which is a low level of inflammation over a longer period of time, and the so-called AGEs, which stand for Advanced Glycation End products, which are proteins, lipids or nucleic acids that have had a sugar molecule attached on itself, which inhibits the function of the molecule. The primary visible signs of aging in the skin are wrinkles, changes in elasticity/texture and uneven skin tone – and ROS are believed to play a role in all of them, which is why antioxidants are believed to be able to delay the aging of the skin. It is therefore intuitive to use antioxidants in e.g. cosmetic products which are applied to the skin and which should be able to neutralize some of the oxidants which are formed in the skin e.g. as a result of the sun's UV rays. There are studies that suggest that topical12 use of some antioxidants can reduce the aging process caused by sunlight – most studies are in vitro studies that have looked at specific cellular processes related to the aging process. There are also in vivo studies with e.g. vitamin C, Resveratrol and extract of green tea (contains polyphenols), which have shown that these can reduce the harmful effects of the sun13. Other studies have shown that such reduction primarily takes place if antioxidants are applied to the skin before uv irradiation and that antioxidants generally work best when several are combined. In vivo studies in humans have also shown that a greater effect is achieved if antioxidants are used both topically and orally at the same time compared to topical or oral use. And it is easier to prevent external signs of aging than to reduce existing signs of aging. Topical use of antioxidants has its limitations, for example in relation to wrinkles, which are mainly formed as a result of changes in the dermis of the skin, which not all antioxidants reach very easily by the topical route. In addition to the antioxidants that can be applied to the skin, there are also the antioxidants that the body itself produces and the antioxidants that are taken orally. The skin is equipped with a network of antioxidants - both those that the body produces and those that are consumed with food and then distributed in the body via the blood. In general, there is a higher concentration of antioxidants in the epidermis compared to the dermis14. And if you look more closely at the very outermost layer of the epidermis, the stratum corneum, you find both water-soluble and fat-soluble and primarily the non-enzymatic antioxidants with the highest concentration in the deeper layers of the stratum corneum. Deeper in the epidermis, you find both the enzymatic and non-enzymatic antioxidants.
Factors with positive signs of aging in the skin
An interesting study has shown that 50-year-old people with a relatively high concentration of antioxidants in the skin have fewer signs of aging compared to people with a relatively low level of antioxidants in the skin. In another observational study with people divided into two groups: those under 45 and those over 45 and a maximum of 55 years at the start of the study; looked at the connection between the intake of foods with a high content of antioxidants and the aging of the skin caused by the sun's rays. Over the 15 years that the study lasted, an increase in skin aging caused by the sun was seen from 42% to 88% overall. People over 45 who ate food with a high antioxidant content had approx. 10% less signs of aging caused by the sun's rays over the 15 years compared to those who ate food with a low content of antioxidants. The same difference was not found for people under 45 years of age. Since a large part of skin aging is caused by the sun's rays, this study suggests that for the mature population, consumption of foods high in antioxidants can have a positive effect on skin aging.
The use of antioxidants today
Antioxidants are found in many different products – both from nature in e.g. food and added to manufactured goods. Food can contain both natural antioxidants and natural and/or synthetic added antioxidants – for example, vitamin E , which is found in many oils, can also be added and is then called E306. Other examples of the natural content of antioxidants in food are vitamin C in oranges and broccoli, the carotenoids beta-carotene and lycopene in tomatoes and Coenzyme Q10 in e.g. meat and nuts. In addition to food, antioxidants - both natural and synthetically produced - are also found in e.g. beverages, nutritional supplements such as vitamin pills, pharmaceuticals, animal feed and cosmetics - and other probably less commonly known areas are e.g. in plastics, as well as in fuel, lubricants, rubber and latex. They are generally added in order to preserve the quality of the product, which can otherwise be degraded by oxidation - e.g. rancidity of oils.
Natural antioxidants
Especially within food and cosmetics, people are gradually moving more towards natural antioxidants (which can both be directly from nature in the form of e.g. extractions from plants or nature-identical synthetically produced antioxidants) - probably especially because some of the synthetically produced man-made antioxidants in studies suggest that they may be harmful at higher consumption levels.
The use of antioxidants within cosmetics
Many different antioxidants are used in cosmetics – both in the form of raw materials containing one specific antioxidant such as vitamin C or coenzyme q10 and in the form of complex antioxidant-rich extracts such as carrot extract, coffee extract, tea extract and many more. They can be added both to maintain the quality of the product and to have an effect on the skin. Antioxidants can be difficult to stabilize, and the biological availability (absorption into the skin) can vary due to the physicochemical properties of the antioxidant. Solutions to these challenges are gradually being developed using different techniques to stabilize and deliver antioxidants and other assets. Normal use of antioxidants in cosmetic products is generally safe and many studies suggest that antioxidants can have more positive effects on the skin. However there is not yet clear clinical documentation that they have the effects that one expects based on the knowledge and theories about oxidants and antioxidants. Future studies must investigate this further.
Sources
Alkadi H. A Review on Free Radicals and Antioxidants. Infectious disorders drug targets. 2020 20(1), 16–26.
Allemann, I. B.; & Baumann, L. Antioxidants Used in Skin Care Formulations. Skin Therapy Letter. 2008 september, vol 13, 7.
Website:
https://www.skintherapyletter.com/aging-skin/antioxidants/
Lokaliseret 25. november 2022.
Birangane, R.S.; Chole, D.G.; Reddy, K.; & Khedkar, S. A Review of Antioxidants. Journal of Indian Academy of Oral Medicine and Radiology. 2011; 23. S351-S353.
Bowe, W. P.; & Logan, A. C. Clinical implications of lipid peroxidation in acne vulgaris: old wine in new bottles. Lipids in health and disease. 2010; 9, 141.
Chen, J.; Liu, Y.; Zhao, Z.; & Qiu, J. Oxidative stress in the skin: Impact and related protection. International journal of cosmetic science. 2021; 43(5), 495–509.
Di Meo, S.; & Venditti, P. Evolution of the Knowledge of Free Radicals and Other Oxidants. Oxidative medicine and cellular longevity. 2020, 9829176.
Garrido-Maraver, J.; Cordero, M. D.; Oropesa-Avila, M.; Vega, A. F.; de la Mata, M.; Pavon, A. D.; Alcocer-Gomez, E.; Calero, C. P.; Paz, M. V.; Alanis, M.; de Lavera, I.; Cotan, D.; & Sanchez-Alcazar, J. A. Clinical applications of coenzyme Q10. Frontiers in bioscience (Landmark edition). 2014, 19(4), 619–633.
Goodarzi, S.; Rafiei S.; Javadi M.; Khadem Haghighian H.; & Noroozi S. A Review on Antioxidants and Their Health Effects. Journal of NItritiun and Food Security. 2018; 3 (2): 106-112.
Hoang H.T.; Moon J.-Y.; & Lee Y.-C. Natural Antioxidants from Plant Extracts in Skincare Cosmetics: Recent Applications, Challenges and Perspectives. Cosmetics. 2021; 8(4):106.
Hoeven, H.; & Prade, H. Aging Better: Clinical Approaches to the Visible Signs of Skin Aging. SOFW Journal – Home & Personal Care Ingredients & Formulations. 2022, 06, 20-24.
Hughes, M. C. B.; Williams, G. M.; Pageon, H.; Fourtanier, A.; & Green, A. C. Dietary Antioxidant Capacity and Skin Photoaging: A 15-Year Longitudinal Study. The Journal of investigative dermatology. 2021; 141(4S), 1111–1118.e2.
Lademann, J. Modern Trends in Sunscreens. Fra A Selection from the Lecture Block of the Cosmetic Science Conference of the DKG e.V. SOFW Journal – Home & Personal Care Ingredients & Formulations. 2022, 1/2, 7.
Lobo, V.; Patil, A.; Phatak, A.; & Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacognosy reviews. 2010; 4(8), 118–126.
Michalak M. Plant-Derived Antioxidants: Significance in Skin Health and the Ageing Process. International journal of molecular sciences. 2022; 23(2), 585.
Oresajo, C.; Pillai, S.; Yatskayer, M.;Puccetti, G.; & McDaniel, D. H. Antioxidants and Skin Aging: A review. Cosmetic Dermatology. 2009; 22 (11), 563-570.
Poljšak, B.; & Dahmane, R. Free radicals and extrinsic skin aging. Dermatology research and practice. 2012, 135206. Rasheed, A., & Azeez, R. F. A. A Review on Natural Antioxidants. Kapitel 5 i C. Mordeniz, Traditional and Complementary Medicine. IntechOpen. 2019.
Reiter, R. J.; Tan, D. X.; Mayo, J. C.; Sainz, R. M.; Leon, J.; & Czarnocki, Z. Melatonin as an antioxidant: biochemical mechanisms and pathophysiological implications in humans. Acta biochimica Polonica. 2003; 50(4), 1129–1146.
Saljoughian, M. An Overview go Antioxidants. U.S. Pharmacist. 2008, 33 (10) HS22-HS28.
Sindhi, V.; Gupta, V.; Sharma, K.; Bhatnagar, S.; Kumari, R.; & Dhaka, N. Potential applications of antioxidants – A review. Journal of Pharmacy Research. 2013; 7, 828-835.
Trüeb R. M. Oxidative stress and its impact on skin, scalp and hair. International journal of cosmetic science. 2021; 43 Suppl 1, S9–S13.
Website:
https://www.hsph.harvard.edu/nutritionsource/antioxidants/
Lokaliseret 25. november 2022.
Wikipedia websites:
https://en.wikipedia.org/wiki/Antioxidant
https://en.wikipedia.org/wiki/Radical_(chemistry)#History_and_nomenclature
https://en.wikipedia.org/wiki/Coenzyme_Q10#Interactions
https://en.wikipedia.org/wiki/Reactive_oxygen_species
https://en.wikipedia.org/wiki/Free-radical_theory_of_aging
Lokaliseret 27. november 2022.
Yadav, A.; Kumari, R.; Yadav, A.; Mishra, J.P.; Srivastava, S.; & Prabha, S. Antioxidants and its functions in human body - A Review. Research in Environment and Life Science. 2016; 9. 1328-1331.
Zhang, S.; & Duan, E. Fighting against Skin Aging: The Way from Bench to Bedside. Cell transplantation. 2018; 27(5), 729–738.




