Vitamin E
Vitamin E
Vitamin E is the primary fat-soluble antioxidant in the body - and has also been shown to have other properties besides being an important antioxidant. It is found in cell membranes and helps prevent the unsaturated fats from being oxidized by free radicals. Vitamin E was discovered 100 years ago and has since been the focus of many studies on various health aspects - from scarring of the skin to cancer and cardiovascular diseases - and for many of them with results which are still being discussed. There is a lot you still do not know about vitamin E - also in relation to its effect on the skin.
PUCA PURE & CARE uses the synthetically produced derivative Tocopheryl Acetate.
PRODUCTS WITH VITAMIN E
VITAMIN E
THE DISCOVERY AND THE CHEMICAL STRUCTURES
In 1922, an unprecedented food factor was discovered that was essential for normal reproduction in rats. This unknown "fertility factor" turned out to be present in e.g. green leafy vegetables, alfalfa leaves, wheat and oats. The substance was isolated in 1935 from wheat germ oil and the empirical formula turned out to be C29H50O2. In the late 1930s, the structural formula - see top structure in Figure 1 - was derived, the substance was synthesized and given the name α-Tocopherol. The name comes from the Greek "tokos", which means birth and "phero" which means to produce and in the end, it got "ol" to make it clear that it is an alcohol (contain an OH group). It was quickly discovered that Tocopherol is an essential antioxidant, and it is mostly this property which vitamin E is recognized for today. In the 1930s and 1940s, the structural formula of 3 other very similar substances was isolated and derived – these had similar antioxidant properties. The 4 Tocopherols were named α (alpha), β (beta), γ (gamma) and δ (delta). It was not until the 1950s and 60s that the structural formulas for a group of 4 other similar substances were discovered and derived: the tocotrienols. Like the Tocopherols, they were given the names α (alpha), β (beta), γ (gamma) and δ (delta). It has since been decided to call all 8 molecules vitamin E - which not all are proponents of, among other things because it has been shown that they have different properties. For example, it has been found that some of the substances can affect different signaling in the cells, gene regulation, membrane processes and nerve functions.
The vitamin E molecules are in general mainly found in vegetable oils and in pure form they are viscous, clear yellow and almost odorless oils. They can be relatively easily oxidized when exposed to heat, light and alkaline conditions. It is therefore in many respects advantageous to stabilize them - for example by placing a chemical group on the O atom in the OH group and thus forming a derivative; the probably most common derivative of Tocopherol is Tocopheryl Acetate.
α-Tocopherol is the most common vitamin E molecule in food and for humans the primary active form. Therefore, most knowledge is on α-Tocopherol and this is often the molecule which is referred to when talking about vitamin E.
All 8 vitamin E molecules α-, β-, γ- and δ-Tocopherol and α-, β-, γ- and δ-Tocotrienols are natural, lipophilic (fat-loving) molecules. The molecules consist of a ring structure and a side chain. It is the structure of the side chain which gives the difference between the Tocopherols and the Tocotrienols - see the two structures in Figure 1. The difference between the α-, β-, γ- and δ-isoforms lies in the location and number of methyl groups (CH3) and hydrogen atoms (H) on the ring structure - see the table under the two structures in Figure 1: There are two locations (R1 and R2) which can be either a methyl group or a hydrogen atom. Figure 1 shows, for example, that the α-isoforms of Tocopherol and Tocotrienol contain a CH3 group in both positions, while the δ-isoforms have H atoms at these two positions and the β- and γ-isoforms have a CH3 group in their respective positions.
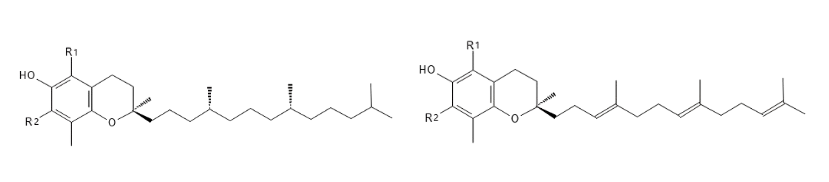
Table header 0
α
β
γ
δ
R2
CH3
H
CH3
H
R1
CH3
CH3
H
H
Figure 1: The structure of RRR-Tocopherol at the top and R-Tocotrienol at the bottom - the table shows the distribution of methyl groups and hydrogen atoms at positions R1 and R2 and thus the structures of the α-, β-, γ- and δ-isoforms of Tocopherol and Tocotrienol.
Figure 1 shows some dotted wedge-shaped bonds and a thicker wedge-shaped bond in each structure. These show the spacious structure of the molecules: dotted wedge-shaped bonds point away from the viewer and thicker wedge-shaped bonds point towards from the viewer. This is called stereoisomerism and means that depending on how the bonds point in the three-dimensional structure, you can have different 2 spacious structures - they are called enantiomers and are designated R and S. Since Tocopherol has 3 places where there may be a difference in the spacious structure, it may occur in 8 different enantiomers: RRR, SRR, RSR, RRS, RSS, SSR, SRS and SSS; while Tocotrienol is only found in 2 enantiomers: R and S. The interesting thing about enantiomers is that they can have different biological activity (1*).
In nature, they are found only in the R-enantiomer, as the enzymes that catalyze biosynthesis ensure that it is precisely this spacious structure which is formed. Thus, the natural α-Tocopherol can be referred to as RRR-α-Tocopherol (it is also referred to as d-α-Tocopherol). When chemically synthesizing α-Tocopherol, all 8 enantiomers can be formed to give an even mix of the 8 enantiomers of α-Tocopherol - such a mix is called all-racemic α-Tocopherol (or dl-α-Tocopherol). It has been shown that the natural RRR-α-Tocopherol is "preferred" by the body, because this has, for example, better affinity for the protein α-TTP in the body, which participates in the uptake.
(1*) A very well-known example of how two enantiomers can have different biological effects is thalidomide: the R-enantiomer had the desired sedative effect, while the S-enantiomer was teratogenic, and was the cause of the many malformed newborns in the late 1950s and early 1960s 'erne.
VITAMIN E
- SOURCES, QUANTITIES AND DEFICIENCY
Vitamins are substances that the body cannot produce itself (a few can be formed in the body, but not in sufficient quantity) and therefore they must be supplied from the outside. Vitamin E is found in many foods. Seeds, nuts, vegetable oils and egg yolk are some of the sources with the highest levels of vitamin E.
Only organisms with the ability to perform photosynthesis - i.e. plants and organisms such as algae and cyanobacteria - can synthesize vitamin E. This is done as mentioned via specific enzymes, which ensure that it is R-enantiomers which are formed. The composition of the different vitamin E molecules varies markedly in different sources. For example, palm, rapeseed, almond, sunflower and especially wheat germ oil contain high levels of α-Tocopherol, while wheat germ oil also contains a lot of β-Tocopherol. γ-Tocopherol is found mainly in corn, rapeseed and soybean oil, while δ-Tocopherol is found in soybean oil, for example. Compared to the Tocotrienols, they are most often found in smaller concentrations, but for example palm and rice bran oil contain relatively much α- and γ-Tocotrienol. There are also more special oils which contain a lot of Tocotrienols and almost no Tocopherols (but in general these oils are not usually used in food).
The plants primarily produce vitamin E due to their antioxidant properties - for example to protect against UV radiation and the lipids in the seeds and kernels against oxidation - rancidity.
Recommended daily intake is slightly different in different parts of the world and different agencies are responsible for the assessments. But generally, around 15 /day is recommended for adults. In addition to the recommended daily intake, one can also talk about the accepted daily intake (ADI). In the EU, EFSA is responsible for assessing food and food additives (as vitamin E is) - in their latest report they have not given an ADI, but a Tolerable Upper Intake Level (UL) of 300 mg/day based on studies on the side effect of vitamin E. JECFA (2*) has derived an ADI - based on human trials and the knowledge that vitamin E is an essential nutrient - of 0.15-0.2 mg/kg/day for dl-a-Tocopherol (all-racemic α-Tocopherol), which corresponds to about 10-15 mg/day for adults.
In general, vitamin E is evaluated to be very safe (very low toxicity) and no undesirable side effects have been documented when consuming Tocopherol through food. Risk of undesirable side effects are present at very high levels of supplementation, where, for example, prolonged blood coagulation time has been seen (tendency to bleed) and at more than 1000 mg/day, evidence has been seen that it can have a pro-oxidant effect. It is known that antioxidants have an antioxidant effect at relatively low concentrations and then some may have the opposite effect (pro-oxidant) at high concentrations.
In different parts of the world, the intake of vitamin E is very different. A larger study has shown that the majority of the earth's population does not reach the recommended intake of 15 mg/day - i.a. because the oils and other foods which are primarily eaten are different in terms of the content of vitamin E and in terms of the composition of the vitamin E molecules. This is important in relation to effect, where it is primarily α-Tocopherol that can be absorbed and can have an effect in the body. For example, corn and soybean oil contains a lot of γ-tocopherol and since these oils are some of the primary oils in the US, they get less absorbed vitamin E than, for example, people in some European countries who eat more sunflower and olive oils which contain more α-Tocopherol.
Vitamin E deficiency symptoms are generally rare, but can occur in premature babies, in people with impaired ability to absorb vitamin E or fats in general, and in people who have a mutation in the gene for the protein involved in the uptake of α-Tocopherol (the protein is called α-Tocopherol Transfer Protein - abbreviated α-TTP).
The symptoms of vitamin E deficiency are primarily neurological dysfunctions such as poor reflexes, lack of control over muscle movements, lack of feeling, poor eyesight, and mental retardation. In addition, a weaker immune system has been seen.
(2*) Joined FAO/WHO Expert Committee on Food Additives
VITAMIN E IN THE BODY
The absorption from the gut seems to be equal for the different vitamin E molecules, but depends on several factors, e.g. the composition of the contents of the intestine (the food one has eaten and other factors). As an example, fiber from food can inhibit the absorption of vitamin E, while fat can promote the uptake. Various studies of how much of the food's vitamin E is absorbed over the intestine range from 20 to 80% - a fairly wide range, which shows that many factors can come into play - including the analysis setup. Orally ingested vitamin E derivatives such as Tocopheryl Acetate (which is common in dietary supplements) are highly hydrolyzed to Tocopherol in the gut before it is absorbed. What is not absorbed through the intestine is excreted in the feces.
From the intestine it passes into the blood and since blood is aqueous and vitamin E is fat-soluble, the transport is some complex. Vitamin E and other lipophilic substances cannot just flow freely in the blood and are therefore transported in the blood in the form of special particulate complexes called lipoproteins. Lipoproteins exist in different sizes and with different qualities. They are usually classified according to their density into e.g. chylomichrons, LDL (Low Density Lipoprotein) and HDL (High Density Lipoprotein) - of which many probably know about LDL and HDL in relation to their importance for cholesterol transport. Lipoproteins generally consist of various fat-soluble substances such as triglycerides, cholesterol, and fat-soluble vitamins and on the surface some more amphiphilic lipids such as phospholipids and also especially proteins which are important for interactions with tissues and other substances in the body.
Via the blood, the lipoproteins with vitamin E come from the intestine to the liver. In the liver, several important and complex processes take place with the vitamin E molecules. In short, the special protein α-TTP (α-Tocopherol Transfer Protein) specifically selects (RRR) α-Tocopherol and helps to ensure that this is re-incorporated into a new lipoprotein and thus is transported from the liver and into the blood again. In this way, it is distributed to the various tissues and can be absorbed there. The other vitamin E molecules (β-, γ- and δ-Tocopherol and α-, β-, γ- and δ-Tocotrienols) do not bind nearly as well to α-TTP and therefore less of these molecules are incorporated in a lipoprotein and sent out into the bloodstream – most of them are instead metabolized more quickly and excreted via the bile (and thus the stool) or via the urine. The natural α-Tocopherol thus has a higher bioavailability than the other Tocopherols and Tocotrienols. It is therefore relevant to look at the distribution of the α-, β-, γ- and δ-isomers when assessing how much effective vitamin E there is in a food source.
In the blood, about 90% of the vitamin E molecules are α-Tocopherol and the concentration is about 25 µmol/L but can be increased up to 2-3 times depending on the intake. However, it must be said that there are different analytical methods for detecting vitamin E - each of which has its advantages and disadvantages - and it is not always easy to compare results.
From the blood, vitamin E is absorbed into the various tissues where they are mainly incorporated into the cell membranes. In addition, vitamin E is stored in adipose tissue - about 90% of the total amount of vitamin E in the body is thought to be in adipose tissue.
Vitamin E is excreted primarily in the urine and before that, it is metabolized to other substances, some of which have been shown to have a biological effect - for example, some metabolites may inhibit the platelet aggregation.
In relation to the skin, vitamin E is also important. It is the most common lipophilic antioxidant in the skin and the level seems to be higher in the epidermis (the upper layers of the skin) than in the dermis. It mainly reaches the epidermis via the sebum of the skin, which is produced in the sebaceous glands and is led out to the stratum corneum (the upper layer of the epidermis) via the hair follicles. From there, it is placed in the extracellular matrix and cell membranes and is thus also a natural part of the skin's surface. It has been shown that it takes at least 7 days before a α-Tocopherol molecule is ingested until it can be found in sebum. Also studies has shown that UV radiation can reduce the amount of vitamin E in the skin (mostly in the stratum corneum) and that the concentration in the epidermis decreases with wounds and with age - which may suggest that supplementation from the outside may be relevant.
Vitamin E and derivatives applied to the skin have been shown to enter the skin, but the amount and how far into the skin they enter depends a lot on the entire composition of the product and in general they do not penetration very deep into the skin but stay primarily in the stratum corneum. For example, a study with pig skin (which is very similar to human skin) and various product formulations have shown that about 6-12% of the content of vitamin E (1% of the formulation) could enter the skin. It is known as mentioned that part of vitamin E on the surface of the skin is oxidized by UV radiation. Therefore, it is sometimes given together with vitamin C, since this can regenerate vitamin E. In addition, you also often make vitamin E more stable by making it a derivative - most often Tocopheryl Acetate or Tocopheryl Succinate, where you have attached an Acetate- or Succinate group on the O atom instead of the H atom in the OH group. There are both advantages and disadvantages to this. The primary advantage is that the molecule is then more stable to the influence of the UV rays, while the primary disadvantage is that vitamin E acts precisely as an antioxidant by being able to release this H atom, which is not present in these derivatives. It could then be advantageous if the derivatives could be metabolized in the skin to the active vitamin E (and thus act as a prodrug), but unfortunately it seems that metabolization in the skin of vitamin E derivatives is generally very low.
VITAMIN E
- FUNCTIONS
Vitamin E is best known for being an essential antioxidant. Antioxidants work by neutralizing free radicals, which are highly reactive substances which can damage e.g. DNA and membrane lipids (primarily the double bonds in unsaturated fatty acids in the lipids) by initiating a radical chain reaction. Usually electrons are in pairs, but in free radicals there is an unpaired electron, which makes the molecule reactive. In the case of vitamin E molecules, they work by donating an electron - or rather: the vitamin E molecule donates the Hydrogen atom from the OH group with one electron. This neutralizes the radical and stops the chain reaction. Such a reaction is called a redox reaction because one molecule (the antioxidant) is oxidized while the other molecule (the radical) is reduced. Now the vitamin E molecule has an unpaired electron (if it is a Tocopherol this molecule is called Tocopheroxyl Radical), but due to the chemical structure it is better at handling an unpaired electron and is therefore not so reactive and at the same time relatively stable. Potentially, the radical vitamin E molecule may react with, for example, another radical vitamin E molecule nearby and form a dimer, or it may oxidize further, or it may react with unsaturated lipids nearby and thereby act pro-oxidant and start a radical chain reaction which it has just prevented. What usually happens is that it reacts with another co-antioxidant, whereby it is reduced (regenerated) back to the original vitamin E molecule so that it can act as an antioxidant again. This other co-antioxidant is often vitamin C (ascorbic acid), which by the reduction of the vitamin E molecule itself is oxidized and thus become a relatively stable radical - this can react with other co-antioxidants such as glutathione. Thus, there is a network of antioxidants which help each other deal with free radicals and the oxidative stress that they form.
The free radicals are formed by many different processes - also completely natural such as metabolism and inflammation, but also via external influences such as UV radiation and pollution (which are the primary factors for the skin). Two main groups among the free radicals are Reactive Oxygen Species, which is abbreviated ROS, and Reactive Nitrogen Species, which is abbreviated RNS. The most common in the body are ROS and it can be quite small molecules such as, for example, superoxide radical anion (O2•⁻), singlet oxygen (1O2, which is not a free radical but can trigger the formation of free radicals) and Hydroxyl radical (•OH) and larger molecules such as lipid-peroxyl radicals (ROO•, where R is the lipid moiety). The various antioxidants have particular affinity for certain radicals; for example, the vitamin E molecules are particularly good at neutralizing (reducing) lipid-peroxyl radicals and singlet oxygen - it of course also plays a role that vitamin E is lipophilic and therefore is in an environment like the cell membrane which will contain the fat-soluble radicals.
The four Tocopherols have similar antioxidant effects in in vitro studies, but since it is α-Tocopherol that is best absorbed into the body, it has the most effect on humans in vivo. Some recent studies shows that the Tocotrienols have a higher antioxidant effect than the Tocopherols, but since the uptake after oral intake is relatively small (as for β-, γ- and δ-Tocopherol) and most foods do not contain much Tocotrienols, it still does not have much effect in the body. However, this can be interesting in terms of topical use of Tocotrienols and in terms of protecting the products themselves with effective antioxidants.
In addition to being an antioxidant, there are studies which suggest that the vitamin E molecules have other properties. For example, cellular signaling in inflammation, where it has been shown that α-Tocopherol can inhibit a number of key steps in this process. There are indications that the way α-Tocopherol functions is by inhibiting the key protein Kinase C enzymes, which play important roles in many different signaling factors such as cytokines, growth factors and hormones. In relation to inflammation, it has been seen, for example, that α-Tocopherol can inhibit platelet aggregation, the production of collagen α1(I) in fibroblasts, the release of certain interleukins and the age-dependent increase in collagenase skin fibroblasts. There are also studies which suggest that vitamin E may affect the regulation of some gene expression and affect the immune system (some studies suggest an inhibitory and others a promoting effect on the immune system).
Vitamin E is thought to play an important role in promoting health, preventing and/or treating some diseases. There are many diseases which are associated with free radicals and oxidative stress and inflammation, so it makes good sense that an antioxidant like vitamin E should have a beneficial effect. And many in vivo studies suggest that there are correlations, but there are also many in vivo studies which do not show a clear correlation. So, for many health aspects, one does not know enough yet to be able to conclude whether vitamin E has a real positive effect. However, in one of the larger studies in humans, it has been shown that higher levels of α-Tocopherol in the blood (30 µmol/L or higher) were associated with low mortality. On the positive end, there is also a meta-study that has tried to find a link between certain inflammatory skin diseases and the level of vitamin E. A correlation was found between lower vitamin E levels in serum in patients with vitiligo, psoriasis, atopic eczema and acne. However, one did not see, for example, a significant difference in the level of vitamin E and the severity of acne. But there are other studies which have not found this connection.
In relation to the skin, there are studies which suggest that vitamin E has a protective effect in relation to damage from UVB radiation - especially with regard to acute redness and signs of aging. In animal experiments, for example, it has been shown that application of α-Tocopherol and α-Tocopheryl Acetate before and just after UV irradiation reduced the damages which UV irradiation would otherwise cause such as oxidative damage to lipids and DNA. In experiments with humans, it has also been shown that vitamin E on the skin can reduce the oxidation of the skin's surface lipids, reduce redness, and inhibit the immune activity which UV radiation can give. Another interesting topic in relation to skin and vitamin E is wounds and scars. One sees a reduction of vitamin E in wounds and vitamin E is therefore often used for wounds and to improve scar formation (both orally and topically). But again, studies do not generally point in the same direction - so despite many studies, there is a lack of conclusive evidence for a real positive effect on wounds and scars.
VITAMIN E
- PRODUCTIONS AND USES
About 35,000 tons of vitamin E are produced each year worldwide. Vitamin E can be made synthetically, semi-synthetically or be extracted from natural oils. Worldwide, the synthetic manufacturing method is most used in relation to e.g. dietary supplements - and often in the form of Tocopheryl Acetate, as it is more stable than Tocopherol. The synthesis itself can take place via various steps and, as mentioned, gives a racemic mix of the Tocopherols. In the semi-synthetic production method, natural vitamin E molecules are extracted and then they are chemically modified - for example, a special chemical reaction can convert a mix of RRR-α-, β-, γ- and δ-Tocopherol into RRR-α-Tocopherol (the most active form). Finally, vitamin E can be extracted from vegetable oils - often from sunflower and soybean oils. The extraction can take place, for example, by molecular distillation. When natural vitamin E is used in cosmetics, it is often extracted from sunflower or soybean oil and typically in a purity of 70-90% RRR-α-, β-, γ- and δ-Tocopherols – the mix depending on the composition of the original oil and the rest is the vegetable oil and maybe a little Tocotrienol. In different connections, it can be an advantage to have vitamin E on a more stable form and possibly a little more soluble in water. That is why, among other things, to make derivatives such as Tocopheryl Acetate and Tocopheryl Phosphate. In addition, nano-emulsions and other types of structures have been made which should help with the absorption and the effect.
Vitamin E and especially RRR-α-Tocopherol is an essential micronutrient which is used in various industries - primarily in medicine, food and cosmetics. As mentioned, it can be regenerated by reacting with vitamin C, so there may be an advantage in combining these two vitamins.
In relation to medical use of vitamin E, many studies have been done on various diseases and the associations with vitamin E level (primarily α-Tocopherol) and in some situations a correlation is found, but the effect of treatment with vitamin E in different studies is often inconsistent. For example, studies have been performed on cardiovascular disease, cancer and cystic fibrosis. In one disease medical treatment with vitamin E is without a doubt effective: this is for those people who have genetic defects in α-TTP. Here, large doses of α-Tocopherol are given to compensate for the lack of functional α-TTP and thus impaired absorption of the vitamin E ingested through food.
Vitamin E is widely used in the food industry and is a very well-researched additive. It is the antioxidant property which are utilized in fatty foods to inhibit oxidation and thus rancidity of the fat. As mentioned, vitamin E is considered to be very safe and therefore there is no reason for concern regarding safety when used as an additive, where it can be listed as one or more of the following E-numbers: E306, E307, E308 or E309. E306 is Tocopherol-rich natural extract (mix of RRR-α-, β-, γ- and δ-Tocopherol and R-α-, β-, γ- and δ-Tocotrienols depending on which edible oil it is extracted from); E370 is synthetic α-Tocopherol (i.e. all-racemic α-Tocopherol); E308 is synthetic γ-Tocopherol (i.e. all-racemic γ-Tocopherol) and finally E309 is synthetic δ-Tocopherol (i.e. all-racemic δ-Tocopherol). Many of the studies behind the assessments are based on the acetate forms and these are therefore also assessed to be safe. In addition to being an additive, vitamin E is also used in various forms as a dietary supplement.
In cosmetics, vitamin E is also used a lot - both to protect the oils and fats in the product and to have an effect on the skin. The concentration varies greatly in different products. Manufacturers of vitamin E suggest using 0.01-0.04% in relation to the fat content of the product to be protected against oxidation (rancidity) - and if there are many polyunsaturated fats, they suggest up to 0.2%, as these are particularly prone to oxidation. The cosmetic product often contains around 0.01-0.5% Tocopherol - and often a slightly higher concentration if, for example, Tocopheryl Acetate is used. It is generally very safe to use in cosmetics. Cases of contact allergy induced by vitamin E and derivatives thereof are found, but the frequency is very low compared to the widespread use.
Vitamin E is without any doubt an important antioxidant and is found naturally everywhere in the body - also in and on the skin. There are many potential effects – many of which there is not yet clear evidence for, but future research will hopefully provide answers.
SOURCES:
Brigelius-Flohé, R.; Kelly. F. J.; Salonen, J. T.; Neuzil, J.; Zingg, J.-M.; Azzi, A. The European perspective on vitamin E: current knowledge and future research, The American Journal of Clinical Nutrition. 2002; Volume 76, Issue 4, Pages 703–716.
De Oliveira Pinto, C. A. S.; Martins, T. E. A.; Martinez, R. M.; Freire, T. B.; Velasco, M. V. R.; & Baby, A. R. Vitamin E in Human Skin: Functionality and Topical Products. I P. Erkekoglu, & J. S. Santos (Eds.), Vitamin E in Health and Disease - Interactions, Diseases and Health Aspects. IntechOpen.
EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), 2015. Scientific Opinion on the re-evaluation of tocopherol-rich extract (E 306), α-tocopherol (E 307), γ-tocopherol (E 308) and δ-tocopherol (E 309) as food additives. EFSA Journal 2015;13(9):4247, 118 pp.
Etsuo N. & Kouichi A. 2019. CHAPTER 1: Vitamin E: Structure, Properties and Functions, in Vitamin E: Chemistry and Nutritional Benefits. Bind 11 af Food, Chemistry Function and Analysis. Royal Society of Chemistry; pp. 1-11.
Fiume, M. M.; Bergfeld, W. F.; Belsito, D. V.; Hill, R. A.; Klaassen, C. D.; Liebler, D. C.; Marks, J. G.; Jr, Shank, R. C.; Slaga, T. J.; Snyder, P. W.; Andersen, F. A.; & Heldreth, B. Safety Assessment of Tocopherols and Tocotrienols as Used in Cosmetics. International journal of toxicology. 2018; 37(2_suppl), 61S–94S.
Kodad, O.; Socias i Company, R.; & Alonso J.M. Genotypic and Environmental Effects on Tocopherol Content in Almond. Antioxidants. 2018; 7(1):6.
Kundu, S.; & Sarkar, D. A Year Away to 100th Year of Vitamin E Synthesis. Journal of Heterocyclic Chemistry. 2021; 58. 1741-1748.
Liu, X.; Yang, G.; Luo, M.; Lan, Q.; Shi, X.; Deng, H.; Wang, N.; Xu, X.; & Zhang, C. Serum vitamin E levels and chronic inflammatory skin diseases: A systematic review and meta-analysis. PloS one. 2021; 16(12).
Michels, A. J.; Traber, M. G.; & Rumbel, H. P. Vitamin E and skin health. Linus Pauling Institute. Written February 2012. Lokaliseret d. 17. Marts 2022: https://lpi.oregonstate.edu/mic/health-disease/skin-health/vitamin-E
Nada, A.; Krishnaiah, Y. S.; Zaghloul, A. A.; & Khattab, I. In vitro and in vivo permeation of vitamin E and vitamin E acetate from cosmetic formulations. Medical principles and practice: international journal of the Kuwait University, Health Science Centre. 2011; 20(6), 509–513.
Packer, L.; Weber, S. U.; & Rimbach, G. Molecular aspects of alpha-tocotrienol antioxidant action and cell signalling. The Journal of nutrition. 2001; 131(2), 369S–73S.
Rizvi, S.; Raza, S. T.; Ahmed, F.; Ahmad, A.; Abbas, S.; & Mahdi, F. The role of vitamin e in human health and some diseases. Sultan Qaboos University medical journal. 2014; 14(2), e157–e165.
Santos, J. S.; Tavares, G. D.; & Barradas, T. N. Vitamin E and Derivatives in Skin Health Promotion. In Erkekoglu, P.; & Santos J. S. (Eds.). Vitamin E in Health and Disease – Interactions. Diseases and Health Aspects. 2021 IntechOpen. https://doi.org/10.5772/intechopen.99466
Sen, C. K.; Khanna, S.; Rink, C.; & Roy, S. Tocotrienols: the emerging face of natural vitamin E. Vitamins and hormones. 2007; 76, 203–261.
Sidgwick, G. P.; McGeorge, D.; & Bayat, A. A comprehensive evidence-based review on the role of topicals and dressings in the management of skin scarring. Archives of dermatological research. 2015; 307(6), 461–477.
Szewczyk, K.; Chojnacka, A.; & Górnicka, M. Tocopherols and Tocotrienols-Bioactive Dietary Compounds; What Is Certain, What Is Doubt?. International journal of molecular sciences. 2021; 22(12), 6222.
Tanaydin, V.; Conings, J.; Malyar, M.; van der Hulst, R.; van der Lei, B. The Role of Topical Vitamin E in Scar Management: A Systematic Review, Aesthetic Surgery Journal. September 2016; Vol 36;8 P. 959–965.
Traber, M. G.; & Stevens, J. F. Vitamins C and E: beneficial effects from a mechanistic perspective. Free radical biology & medicine. 2011; 51(5), 1000–1013.
Wikipedia website. https://en.wikipedia.org/wiki/Vitamin_E & https://en.wikipedia.org/wiki/Tocopherol Located 17. Marts 2022.