Hyaluronic Acid
Hyaluronic Acid
Hyaluronic acid and its sodium salt Sodium Hyaluronate are important components in the body and are found almost everywhere in the body.
The molecular weight is crucial for its properties. Overall, high molecular weight hyaluronic acid has anti-inflammatory and immunosuppressive properties and is involved in certain gene expressions, while low molecular weight hyaluronic acid has shown antioxidant, inflammatory and immunostimulatory properties.
Fælles er det at hyaluronsyre er utrolig god til at binde vand og dermed give fugt og fylde til fx huden. Fugt er en helt grundlæggende komponent i huden og ofte en del af løsning til at bevare en pæn og funktionel hud. Konsekvenserne af reduceret fugt i huden kan være mange – fx tør hud som har en dårligere barriere og dermed øget risiko for infektion. Tør hud øger også risikoen for allergi og heling af huden forringes, når der er mindre fugt. I mange hudproblemer er der er korrelation med manglende fugt. Ældre hud er også kendetegnet ved at indeholde mindre fugt.
PUCA PURE & CARE uses a Sodium Hyaluronate extracted from Steptococcus bacteria. It is a mixture of 10% low molecular weight and 90% medium molecular weight.
PRODUCTS WITH HYALURONIC ACID
HYALURONIC ACID
A SIMPLE BUT INTERESTING CHEMICAL STRUCTURE
Hyaluronic acid is a naturally occurring glycosaminoglycan - a biopolymer of disaccharides with very special physical, chemical and biological properties. Its properties are highly dependent on the molecular weight, which can range from 10 to 1000 kDa (1). Thus, the name hyaluronic acid, or hyaluronan as it is also called, covers not only one molecule with one particular structure, but all the chain lengths that occur with this specific and repeating structure of the two sugar molecules D-glucuronic acid and N-acetyl-D-glucosamine - See Figure 1.
Because hyaluronic acid spans such a wide range of molecules, they are often divided into some groups based on their molecular weight. There is no complete agreement on where the limits for each group go, but it is roughly as follows: 40-500 kDa is low molecular weight (LMW), 500-2000 kDa is medium molecular weight (MMW) and> 2000 kDa is high molecular weight (HMW).
The structure of hyaluronic acid is a twisted chain without branches and consists of between 500 and 50,000 monosaccharides and measures up to 10 nm - however, usually about 1 nm (2). These are relatively large molecules. By comparison, a strand of hair is about 80,000 to 100,000 nm in diameter.
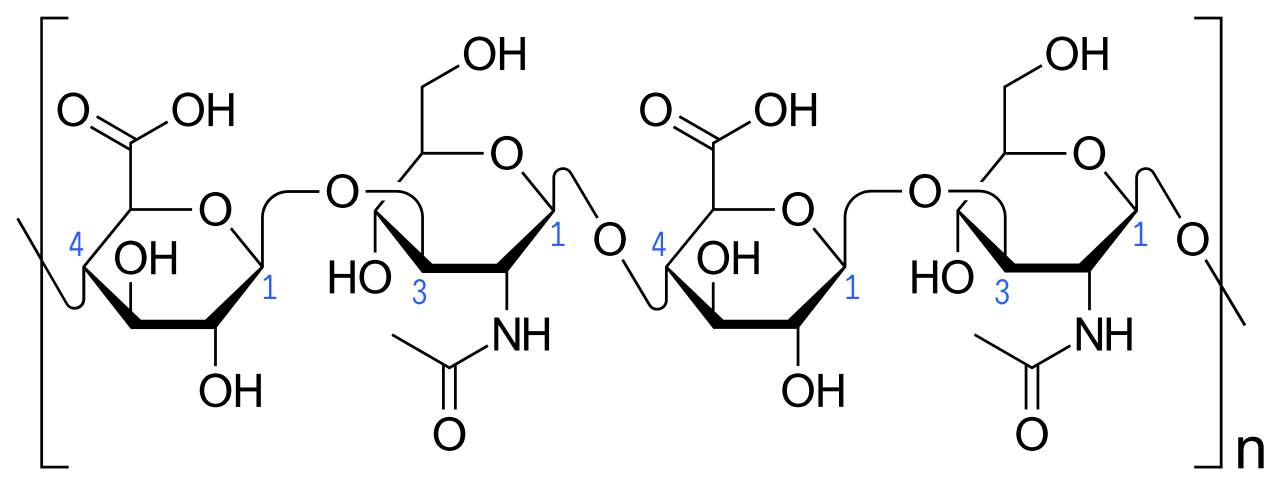
Figure 1 Hyaluronic acid structure. Here are two units of the disaccharides that make up the molecular unit of hyaluronic acid. On the left side of the figure is a D-glucuronic acid, which is bound to an N-acetylglucosamine with a 1 → 3 glycoside bond. This N-acetylglucosamine is bound to the next d-glucuronic acid with a 1 → 4 glycoside bond, which is bound together with the last acetylglucosamine in the figure via a 1 → 3 glycoside bond. The small "n" means that there can be many repetitions of this structure.
Hyaluronic acid, unlike the other glycosaminoglycans, such as chondroitin sulphate, dermatan sulphate and heparin, is not sulphated - i.e. there is no sulfate group in it and moreover, hyaluronic acid, unlike the other glycosaminoglycans, is not biosynthesized inside the cell's Golgi apparatus, but by proteins located in the cell membrane. Glycosaminoglycans have many different functions in and around the cells - for example, some are involved in regulating cell growth, vascular formation, various neurological processes, and infection.
The name "hyaluronan" was introduced in 1986 to adapt the original name, hyaluronic acid, to the current polysaccharide nomenclature. But today the name hyaluronic acid is often used - the corresponding sodium salt is sodium hyaluronate, where the hydrogen atom in the D-glucuronic acid part is replaced with sodium, which is simply loosely bound and thus the molecule is in anion form - i.e., it is negatively charged. In the body, hyaluronic acid is primarily in anion form. In the INCI nomenclature it is "Hyaluronic Acid" and "Sodium Hyaluronate" and it is most often these two versions that are used in the many different applications.
(1) kDa = kilo Dalton (1000 Da). Unit of mass, which corresponds to g / mol and is used to express how much a molecule weigh is. For example, one water molecule weighs about 18 Da.
(2) nm = nanometer. 1 nm = 0.0000001 cm
HYALURONIC ACID
- OCCURRENCE, BIOSYNTHESIS AND DEGRADATION
Hyaluronic acid was first isolated from cattle eyes in 1934 - today it is known that it is found almost everywhere in the human body, and it is the same structure that is found in most animals and even in some bacteria. The chemical structure of hyaluronic acid was identified in the 1950s.
The highest concentration of hyaluronic acid in humans is found in the eye and synovial fluid and cartilage, but the largest amount is found in the skin - here about 50% of the body hyaluronic acid is located, primarily in the dermis layer of the skin. More specifically, hyaluronic acid is found especially in the extracellular matrix around the cells, and constitutes a primary component together with, for example, collagen and other glycosaminoglycans in the supporting and protecting structures around the cells.
Something very special about hyaluronic acid is that it can bind a lot of water and is thus very important in terms of retaining moisture and fullness in the tissues. It has been discovered that in young skin it is mostly hyaluronic acid in "free form" which can bind a lot of water, while in older skin it is more bound to proteins and other structures and thus has less water-binding capacity.
A person weighing 70 kg contains about 15 g of hyaluronic acid and about 1/3 of this is converted every day - i.e. hyaluronic acid has a very high turnover: a large part is broken down and built up every day.
The biosynthesis of hyaluronic acid is controlled by Hyaluronan synthase proteins (abbreviated HAS), of which there are three types in mammals: HAS-1, HAS-2 and HAS-3, each of which synthesizes different lengths of hyaluronic acid. These proteins are transmembrane, which means that they are located in and span the entire thickness of the cell membrane. Here they make sure that the synthesis itself takes place on the inside of the cell while the growing chain eventually emerges on the outside of the cell.
Most cells in the body have the ability to synthesize hyaluronic acid. In the skin, it is primarily the fibroblasts that are responsible for the synthesis, which is increased by wound healing, for example.
The rate of breakdown of hyaluronic acid is not the same in different parts of the body. For example, hyaluronic acid has a half-life of 2-5 minutes in the blood, about 1 day in the skin, 1-3 weeks in cartilage and about 10 weeks in the eyes. The degradation process can be both via enzymes and via free radicals (oxidation) - but it is difficult to study and one does not quite know how the distribution is between these two types of processes. The enzymes that are responsible for the breakdown are called hyaluronidases (abbreviated HYAL) - there are at least 7 types of hyaluronidase-like enzymes, of which HYAL-1 is mainly found in serum. The degradation products are oligosaccharides and low-molecular hyaluronic acid. The decomposition via free radicals is an oxidation process which, for example, takes place at UV exposure and at low and high pH.
Hyaluronic Acid
- A MOLECULE WITH MANY FUNCTIONS IN THE BODY
Hyaluronic acid is very hygroscopic - i.e. it has a very large capacity to bind water - and this is a property that gives hyaluronic acid several of its functions. It has been measured that hyaluronic acid can bind about 1000 times its own weight in water. The higher the molecular weight, the higher the water-binding property. This makes hyaluronic acid a very good moisture binder and as it binds so much water it also gives volume. In addition, it provides a lubricating effect and viscosity. Just 1% HMW hyaluronic acid in water gives a rather viscous gel with special rheological properties: It is pseudo-plastic and viscoelastic, which means that the viscosity decreases when stress or pressure is applied to the and it has a certain elastic ability, which is important for its lubricating, stabilizing and shock-absorbing effect. For example, in joints and other places in the body where there is movement, the lubricating effect is important.
At the cellular level, hyaluronic acid helps regulate the migration of cells - the hydrated matrix that hyaluronic acid provides facilitates cell migration, which is important in many processes in the body. For example, in wound healing and in the formation of new blood vessels and the immune system is very dependent on cells being able to move around in the tissues. Likewise, a large cell migration occurs during cancer development.
A number of studies have been carried out on the role of hyaluronic acid in the wound healing process, which is a very complex process, with many cellular and molecular "actors". The wound healing process can be divided into several (partially overlapping) phases: hemostasis, inflammation, cell proliferation and remodeling. The hemostasis is about stopping the bleeding - the blood coagulates. In the inflammation phase, the area is “cleansed”, which means damaged and dead cells and any external units (e.g. bacteria) are cleaned out. This phase includes, for example, white blood cells. Special growth factors are instrumental in starting the next phase:
cell proliferation. Here cells grow and divide so that it fills the tissue with the right cells and new blood vessels are formed and more. The last phase - remodeling - involves tissue "maturation" and making adjustments. For example, some of the collagen that is formed is replaced and rearranged, which increases the tensile strength of the tissue, and used components and cells are removed.
There are particularly high concentrations of high molecular weight hyaluronic acid at the beginning of the healing process. The cells in the area will naturally synthesize more hyaluronic acid upon damage to the skin, where it forms a moist and gel-like medium in which the various cells can more easily migrate. Gradually, hyaluronic acid is broken down by hyaluronidase, which is secreted by the cells that migrate into the medium. This results in hyaluronic acid of smaller molecular weight, which promotes inflammation and blood vessel formation. In this way, hyaluronic acid plays different roles in the wound healing process and helps to control the phases.
Low molecular weight hyaluronic acid has shown antioxidant, inflammatory and immunostimulatory properties, while high molecular weight hyaluronic acid is involved in certain gene expressions and has anti-inflammatory and immunosuppressive properties. In the dermis of the skin, it helps to regulate the water balance and stabilize the skin structure and stimulate collagen synthesis in fibroblasts.
It is also known that hyaluronic acid can bind certain receptors - e.g. CD44, which is found on most cell types and is involved in the regulation of adhesion, migration, activation and differentiation of cells and the cancer metastasis process. CD44 is also involved in regulating hyaluronic acid levels. Another type of receptor that hyaluronic acid can bind is RHAMM, which is also involved in cell growth and migration.
Hyaluronic Acid
- MANUFACTURING AND USES
As mentioned, hyaluronic acid is found in many places - in addition to humans, animals, and bacteria, it is also found in many different plants. Particularly high concentrations in the human body are found in the umbilical cord, synovial fluid, skin and vitreous of the eye, but the highest concentration among all animals is found in rooster combs. In relation to industrial use, there are generally three manufacturing methods: extraction from animal tissue, bacterial production, and in vitro enzyme production.
For extraction from animal tissue, rooster combs, human umbilical cords, bovine eyes and synovial fluid from cattle have primarily been used. Today, predominantly rooster combs are used for this manufacturing method - and it is widely used - especially for medicinal use of hyaluronic acid. The advantages of this manufacturing method are that it is very well known and the materials to be used are generally quite inexpensive (food production residues), it is naturally produced in the tissue and one can extract hyaluronic acid with relatively high molecular weight and very pure. The disadvantages are that there is a risk of contamination with proteins, nucleic acids and viruses, the yield is not so great, and the purification process must be extensive with the risk of degrading the hyaluronic acid polymers.
Production via bacteria started in the 1960s - especially when it was discovered that hyaluronic acid from animal materials can contain unwanted proteins. Today it is - in relation to cosmetics - the most widely used production method. There are many bacteria that can produce hyaluronic acid and secrete it on the outside of the cell wall, from which it is relatively easy to "harvest" it and which by the way also makes the bacteria less easy to detect for the immune system, as hyaluronic acid is completely identical in humans and these bacteria. The bacteria that are primarily used today are strains of Streptococcus, in addition, Escherichia coli, Lactococcus lactis and Bacillus subtilis are also used. The advantages of bacterial production are that the technique is mature and well known, it is relatively easy to get a high yield and reasonably high molecular weight and very pure. It is also possible to influence how much hyaluronic acid the bacteria produce. The disadvantages are that it may be GMO bacteria that are used and there is a risk of contamination with endotoxins, proteins, nucleic acids, and heavy metals.
Finally, there is the newest production method: Enzymatic production, in which enzymes from bacteria are used to synthesize hyaluronic acid in vitro. The advantages of this newer versatile method are that it is easier to control the molecular weight, there is no risk of contamination, and the quality can be more easily controlled. The disadvantages are that it is a method that is still under development, it is relatively expensive.
The hyaluronic acid is in some cases modified by inserting crosslinks into the molecule to make it more stable.
By virtue of the many different properties, hyaluronic acid is used for several different purposes, especially in medicine, dietary supplements, and cosmetics. A very important factor for its use is that hyaluronic acid is biocompatible and generally very safe to use on and in humans.
For medical purposes, hyaluronic acid is used for e.g. wound healing in the form of a wound dressing in film form, which can promote wound healing by providing a moist environment in the wound. It is used during eye surgery (e.g. injected into the eye to preserve the shape of the eye) and in eye drops and artificial tears to relieve dry eyes. Another area of medicine where hyaluronic acid is used is in arthritis and osteoarthritis - especially in the knees. Injecting hyaluronic acid solution into the knee joint can reduce pain. There are probably several mechanisms of action behind the analgesic effect. For example, it has been shown that hyaluronic acid can promote the synthesis of components for cartilage matrix, which is characterized by being broken down in osteoarthritis, and inhibit the breakdown of the same and reduce inflammation - in addition to providing a shock-absorbing effect and moisture to the joint. In osteoarthritis, you typically also have less hyaluronic acid in the joint, so injection replaces some of what was lost. However, as described, hyaluronic acid degrades relatively quickly. Hyaluronic acid with crosslinks is more stable and can last a little longer than the all-natural ones. But very interesting the analgesic effect of injecting hyaluronic acid lasts for longer than the hyaluronic acid molecules themselves do in the tissue - perhaps due to a stimulation of hyaluronic acid formation and an anti-inflammatory effect. A few other interesting areas in medicine are that hyaluronic acid can be used to target the medicine to the right place in the body - for example in cancer treatment. And finally, degradation products of hyaluronic acid can be used as a biomarker for certain diseases at an early stage.
In cosmetic surgery, hyaluronic acid is used as a filler, where hyaluronic acid (possibly stabilized with crosslinks or otherwise) is injected into the skin to give fullness and smooth out wrinkles. The effect typically lasts around ½-1½ years, and it is generally a very safe treatment. The most common side effects are pain, redness and itching.
Hyaluronic acid is also used in supplements where it has shown some but not very strong evidence for effect against osteoarthritis. However, it is shown that orally ingested hyaluronic acid is absorbed and distributed in the body.
In cosmetics, hyaluronic acid is a rather desired component - especially due to its moisturizing properties. Moisture is a very basic component of the skin and often part of a solution to maintain a nice and functional skin - and to relieve more skin problems. As with the other target areas, the molecular size of hyaluronic acid is important for its properties relative to skin. In general, hyaluronic acid with a high molecular weight will not enter the skin but provide a protective film over the skin - and thereby provide moisture. Hyaluronic acid with a low molecular weight will more easily be able to get a little into the skin and bind moisture there. Many studies have been done with hyaluronic acid in cosmetics. One study, for example, has shown that 0.1% low molecular weight hyaluronic acid (50-130 kDa) was better at reducing wrinkles around the eyes and improving the moisture level and elasticity of the skin, while higher molecular weight hyaluronic acid generally also has positive effects in slightly less degree.
The concentration of hyaluronic acid in cosmetics is usually below 1%. In addition to its effect on the skin, it also has a thickening and moisturizing property in the product itself. It is most often Sodium Hyaluronate, which is used in cosmetics.
SOURCES:
Becker, L.; C., Bergfeld, W. F.; Belsito, D. V. et al. Final Report of the Safety Assessment of Hyaluronic Acid, Potassium Hyaluronate, and Sodium Hyaluronate. International Journal of Toxicology. 2009; 28(4_suppl), 5–67.
Boeriu, C.; Springer, J.; Kooy, F.; Broek, L. & Eggink, G. Production Methods for Hyaluronan. International Journal of Carbohydrate Chemistry. 2013. 14.
Bukhari, S.; Roswandi, N. L.; Waqas, M. et al. Hyaluronic acid, a promising skin rejuvenating biomedicine: A review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects. International journal of biological macromolecules. 2018; 120: 1682–1695.
Gupta, R. C.; Lall, R.; Srivastava, A. & Sinha, A. Hyaluronic Acid: Molecular Mechanisms and Therapeutic Trajectory. Frontiers in veterinary science. 2019; 6, 192.
Necas, J.; Bartosikova, L.; Brauner, P. & Kolář, J. Hyaluronic acid (Hyaluronan): A review. Veterinarni Medicina. 2008; 53 (8).
Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Dermato-endocrinology. 2012; vol. 4,3: 253-258.
Pavicic, T.; Gauglitz, G. G.; Lersch, P. et al. Efficacy of cream-based novel formulations of hyaluronic acid of different molecular weights in anti-wrinkle treatment. Journal of drugs in dermatology. 2011; 10(9): 990-1000.
Salwowska, N. M.; Bebenek, K. A.; Żądło, D. A. & Wcisło-Dziadecka, D. L. Physiochemical properties and application of hyaluronic acid: a systematic review. Journal of cosmetic dermatology. 2016; 15(4), 520–526.
Saranraj, P. Hyaluronic Acid Production and its Applications - A Review. International Journal of Pharmaceutical & Biological Archive. 2013; 4 (5): 853-859.
Stern, R. & Maibach, H. I. Innovations in Hyaluronic Acid. Cosmetics & Toiletries magazine – A dermatological View. 2013 March Vol 128, No 3.
Vasvani, S.; Kulkarni, P. & Rawtani, D. Hyaluronic acid: A review on its biology, aspects of drug delivery, route of administrations and a special emphasis on its approved marketed products and recent clinical studies. International journal of biological macromolecules. 2020; 151, 1012–1029.
Walker, K.; Basehore, B.M.; Goyal, A. et al. Hyaluronic Acid. Opdateret 15. November 2021 i StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; Januar 2022.
Wikipedia webside. Hyaluronic acid. Lokaliseret 29. Januar 2022: https://en.wikipedia.org/wiki/Hyaluronic_acid
Wikipedia webside. Wound healing. Lokaliseret 31. Januar 2022: https://en.wikipedia.org/wiki/Wound_healing