Glycerin
Glycerin
Glycerin is a small, natural molecule with excellent moisture-binding properties. It is one of the most used ingredients in cosmetics and has been shown in many studies to have several positive properties for the skin. Glycerin is one of the skin's humectants, which helps to keep the skin in the right moisture balance, where the skin's many functions function optimally.
PUCA - PURE & CARE uses glycerin extracted from vegetable oil with a purity of over 99.5%.
PRODUCTS WITH GLYCERIN
THE STRUCTURE OF GLYCERIN
Glycerin is a natural molecule with quite remarkable properties. In its pure form it is a colorless, odorless, viscous, slightly sticky, sweet-tasting, and hygroscopic liquid with a fairly high density. The special structure means that there are both inter- and intra-molecular hydrogen bonds, which gives it a very high boiling point. The molecule is very hydrophilic and can thus be easily mixed with water and its hygroscopic properties means that it can bind an amount of water corresponding to its own weight.
Glycerin is a very simple molecule consisting of 3 C atoms in a row, with an alcohol (OH) group on each C atom - it is a small polyol and these are known to be good at binding water, as alcohol groups and water molecules like to interact. The name "Glycerin" is the INCI name and the name most commonly used commercially, while "Glycerol", which is derived from the Greek "glukerós" and means "sweet", is a more chemical name for the same substance. The IUPAC(1) name is propane-1,2,3-triol, which more clearly describes the chemical structure - see Figure 1.
Glycerin was accidentally "discovered" as a chemical by a Swedish-German researcher in 1779, when he performed a chemical reaction between olive oil and lead monoxide, from which he discovered this water-soluble substance with a sweet taste. But glycerin has been "known" since 2800 BC, when soap was made by heating fat with ash, whereby glycerin was split off. In 1836 the empirical formula was proposed to be C3H8O3, but it was not until 1886 that the structural formula was determined, as shown in Figure 1. Before that, in 1866 Alfred Nobel had found a method to stabilize nitroglycerin and thereby he invented the dynamite. Nitroglycerin is a very explosive substance, which is produced by putting a nitrate group on each of the three OH groups in glycerin - it was by this discovery that glycerin became economically and industrially interesting.

Figure 1 The chemical structure of Glycerin.
This small molecule is found everywhere in nature - especially as part of fat molecules. Most fats - both animal and vegetable - are triglycerides, which as the name indicates, contains glycerin. It can be said that the glycerin molecule is the "backbone" and the three "arms" that attach to it are fatty acid molecules, each of which is bound to one of the three alcohol groups via an ester bond - see Figure 2. Degradation of such a triglyceride gives 3 free fatty acids and a glycerin molecule. Both degradation and build-up of triglycerides occur naturally in living organisms. Regarding ingestion of glycerin, it can be mentioned, for example, that wine, beer and other fermented products contains glycerin in free form.
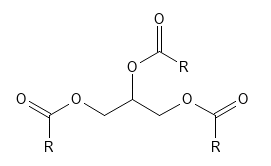
Figure 2 Triglyceride - the basic chemical structure of most fats. "R" represents the carbon chain in each of the three fatty acid moieties - these may be the same or different and may, for example, contain double bonds, giving an unsaturated fatty acid.
(1) IUPAC = International Union of Pure and Applied Chemistry. An international organization, which i.a. has prepared a special nomenclature for chemical substances.
THE SKIN
- STRUCTURE, RENEWAL AND THE IMPORTANT WATER
In order to understand the importance of glycerin and how it acts in the skin, it is a great advantage to know how the skin is structured – see figure 3 - and how the top layer is continually renewed.
The skin generally consists of three layers: At the bottom is the subcutis - also called the hypodermis, which consists primarily of lipids and connective tissue. In the middle is the dermis, which mainly consists of connective tissue in which, for example, nerve endings, blood vessels, hair follicles, sebaceous glands and sweat glands are embedded. At the top is the epidermis, which consists of several layers: At the bottom is the stratum basale, which is a single cell layer of i.a. melanocytes, undifferentiated keratinocytes and stem cells that constantly form new keratinocytes (cells). These keratinocytes migrate outwards and gradually form the other epidermal layers, which are: Stratum spinosum, stratum granulosum, stratum lucidum and finally the outermost layer is the stratum corneum (SC), which is about 10-30 µm thick. The stratum corneum contains several layers of primarily dead, flat keratinocytes, which are called corneocytes - these are embedded in a special lipid lamellar structure, which consists of various fats, which is an important element in the skin barrier. Stratum corneum is often described as a masonry of bricks (the corneocytes) and mortar (intercellular lipid structure). From the stratum corneum surface there is constantly shedding of the outermost layer of skin cells so that the skin is renewed - this process is called "desquamation" - peeling. The process is usually well regulated and important for e.g. the skin's appearance and mechanical properties.
This turnover from being a new living keratinocyte near the stratum basal to become a dead corneocyte, which is shed from the stratum corneum takes approx. 4 weeks - but is for example slower in older skin and faster in psoriasis skin. The epidermis does not contain blood vessels and thus especially the lower layers of the epidermis are dependent on nourishment from and waste delivery to the blood in the dermis. At the junction between the dermis and the epidermis is a membrane layer called the Dermal-epidermal Junction (DEJ) which consists of special protein structures. In young skin, this transition is very wavy - the dermis forms papillae, which forms elevations and depressions in the transition - so that there is a large surface between the dermis and the epidermis, where the nutrient supply can take place. In older skin, this transition gradually flattens out more, giving a smaller surface for nutrient supply to the epidermis. In addition, this transition is relatively impermeable to e.g. glycerin.
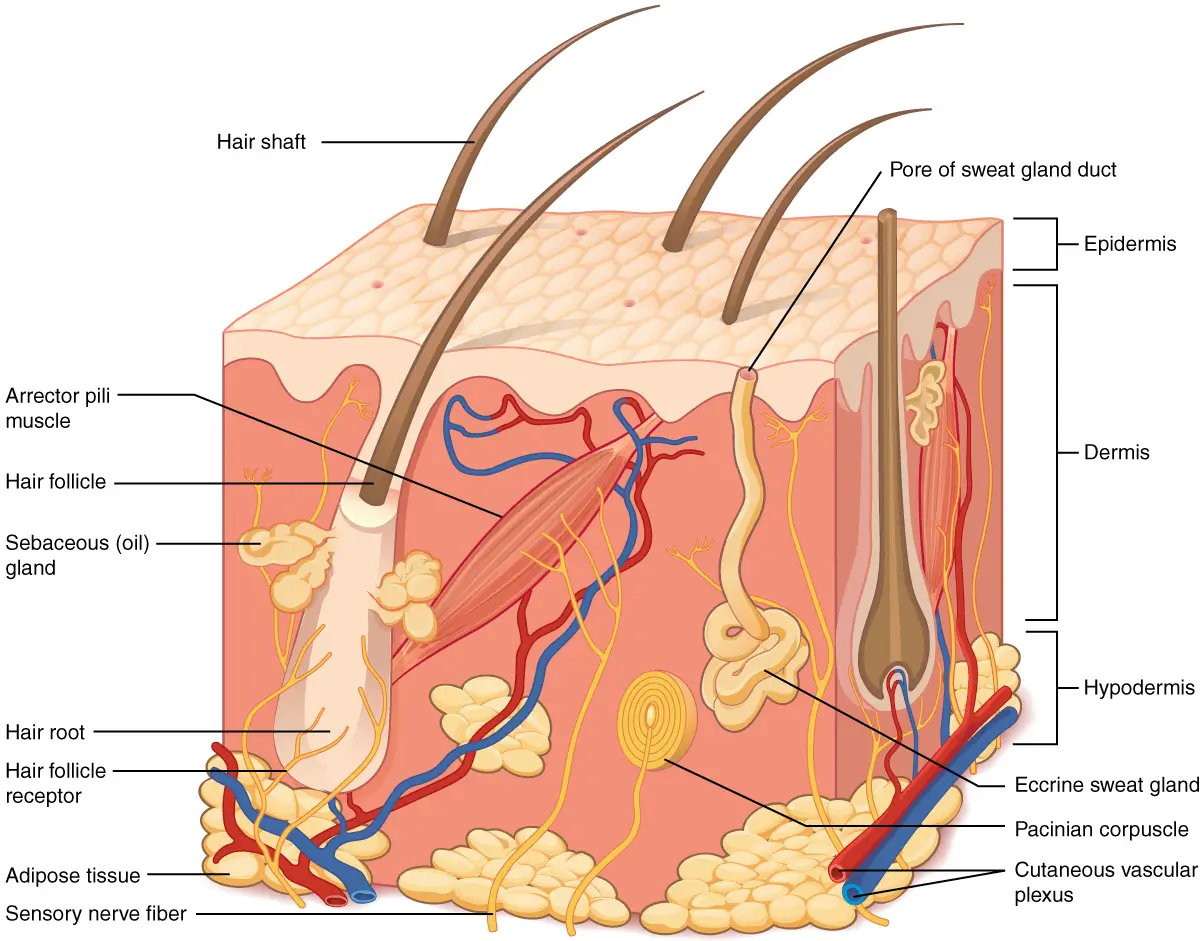
Figure 3 The structure of the skin with the three layers; epidermis at the top (which consists of several layers), dermis in the middle and hypodermis at the bottom. Note the wavy transition between the dermis and the epidermis - it is the so-called "Dermal-epidermal Junction", whose wavy shape comes from dermal papillae. Also note the sebaceous glands, which are connected to the hair follicles - this is where sebum is formed, which is released to the skin via the channel in the hair follicle. This figure is from Wikipedia.
Moisture and hydration - expressions of the water content, which is an incredibly important factor for the skin. Water plays a crucial role for the skin's many physiological and mechanical functions and for the appearance of the skin - both too much and too little water can change the skin's properties, so it is important to have the right balance, but most often it is dehydration that is the problem when the skin has an imbalance in the water level. Water is a plasticizer and essential for the function of certain enzymes. For example, the enzymes that participate in the desquamation process. If there is not high enough moisture and this process thus does not take place at the right pace on the surface of the stratum corneum, it results in the retention of corneocytes, which should be shed, thus forming small flakes, which characterizes dry skin.
Overall, the water level in the different layers of the skin depends in particular on how permeable the layers are to water. That is, how easily water moves through the layers in both directions - both from the outside and in and from the inside out. And how good the skin is at retaining the water. The following are important factors for these properties:
- The presence of hyaluronic acid and glycerin in the dermis and epidermis. In relation to the epidermis, a very clear correlation has been found between the moisture level and the level of glycerin. These molecules are both important hydrating agents that can hold on to the water, keep the environment moist and inhibit the evaporation that takes place all the time from the skin. This evaporation is often called "TEWL", which stands for Trans Epidermal Water Loss. Measurement of TEWL is often used to assess the barrier function of the skin.
- The presence of the so-called Natural Moisturizing Factors (NMF), which are small natural hygroscopic molecules - moisturizers like glycerin and hyaluronic acid. NMF generally consists of a mix of specific salts such as lactates, urea, electrolytes and about half of it are amino acids and derivatives of amino acids. These amino acids come from the moisture-regulated proteolysis (degradation) of proteins - primarily filaggrin. It is thus a smart feedback mechanism, which controls whether more NMF is needed. Some also count glycerin as an NMF.
- The presence of "tight junctions" in the epidermis - these are especial protein structures which form a watertight barrier between the cells - and thus inhibit evaporation.
- The presence of certain transport channels called “aquaporins” - especially aquaporin-3 has been shown to be crucial for the hydration of the epidermis - more on this in the next section.
- The organization of the intercellular lipid structure in the stratum corneum - this forms a very important part of the skin's barrier to water loss. These lipids consist primarily of ceramides, cholesterol, and fatty acids, which are influenced by genetics, age, nutrition and the environment, for example.
- The spatial organization of the corneocytes in the epidermis.
- The production of sebum from the sebaceous glands shows in some studies a correlation with the moisture in the stratum corneum.
GLYCERIN IN THE BODY
- FOCUS ON THE SKIN
Glycerin occurs naturally in the body - for example, the concentration of glycerin in the blood serum is usually 0.46 and 18.5 mg / L. However, the greatest amount of glycerin is found in the form of an important component in most of the fat found in the body. In addition to the mentioned triglycerides, which i.a. one of the body's natural energy reserve and isolation; glycerin is also a part of phospholipids, which are the primary fat in all cell membranes. These are the same types of fat molecules that are found in animals and plants and thus you also ingest a lot of glycerin through food. Fat from the food will be hydrolyzed in the intestines so that glycerin and fatty acids are released and can be absorbed. For glycerin, the absorption takes place fairly quickly into the blood and from there on to the liver, where it can be further metabolized via various steps - to e.g. glucose and from there to glycogen or to fats. It can also be metabolized to CO2, which is the way glycerin is primarily excreted - via the exhaled air. A very small portion is excreted in the urine. Since the liver is the primary site where glycerin is metabolized, measurement of glycerin in the liver can be used to assess the condition of the liver.
The glycerin that is in the blood is distributed throughout the body, where it i.a. can be absorbed into the skin - this is together with the glycerin that is released in e.g. the dermis the endogenous glycerin, which has been shown to be crucial for the skin's moisture level. In addition, there is the (exogenous) glycerin, which can be applied to the skin. Glycerin is a very small molecule and has been shown to enter the epidermis from the outside and have a beneficial effect there. It can form a small reservoir and bind water in the stratum corneum and can thus supplement the beneficial effect of the endogenous glycerin - and possibly. compensate for the lack of endogenous glycerin in the stratum corneum, which usually contains relatively much glycerin.
In addition to being a very small molecule, glycerin is also water soluble. It has been shown that regular washing and immersion of the skin in water removes some of the glycerin that is on the skin and can thereby reduce the moisture in the stratum corneum. In normal-functioning skin, glycerin levels and moisture will return to normal levels within a few hours - this shows that glycerin mainly comes from inside and especially from sebaceous glands, as areas with many sebaceous glands return to normal faster than areas with few sebaceous glands. In the sebaceous glands, where sebum is produced, there are lipase enzymes that break down fats (triglycerides) and thus release glycerin. The sebaceous glands have, as Figure 3 shows, connection with and outlet in the hair follicles located in the dermis. Lipase enzymes are also found in and on the epidermis, but studies do not suggest that these contribute much to the generation of the glycerin found in the epidermis. There are thus primarily two sources of glycerin in the skin: Serum in the blood, which flows through the dermis and sebum from the sebaceous glands.
A very important factor in getting glycerin from the dermis to the epidermis is the mentioned aquaporins - and especially aquaporin-3. Aquaporins are a family of membrane-bound transport proteins that form small channels through membranes. As the name suggests, they generally transport water, but some of them can also transport other small molecules - such as glycerin. Aquaporins are found in many places in nature - for example in plants, insects and larger animals. In humans, they are found in many different locations in the body - for example in the kidneys, where they are important for urine formation and in the brain and eyes, where water balance is important. There are 13 types of aquaporins, of which aquaporin-3 in particular has an important role to play in the skin. This has i.a. been found out by examining mice that lacked the gene for aquaporin-3. These mice had about three times less water in the stratum corneum and about half as much glycerin in the epidermis compared to ordinary mice. The level of glycerin in the dermis was normal. In addition, these mice had less elastic skin and the skin was slower to heal and recover the barrier. Something very interesting from these studies was that topical administration of (exogenous) glycerin could counteract the reduction in moisture level that the mice had. From these studies it can be deduced that aquaporin-3 facilitates glycerin transport into the epidermis (through the otherwise for glycerin rather impermeable layer between the dermis and epidermis) and that glycerin is a crucial component in the skin's moisture level, elasticity, and barrier reconstruction. Aquaporin-3 is therefore also called aquaglyceroporin - as this type of aquaporin can transport both water and glycerin across membranes. It has been shown that the concentration of aquaporin-3 reflects the level of water in the different layers of the epidermis. In healthy epidemics, aquaporin-3 is found mainly in the stratum basale (the lower part of the epidermis closest to the dermis, which contains blood vessels) and the concentration of aquaporin-3 decreases as one moves outwards in the epidemic layers. It correlates with the water content, which is about 75% in the lower part of the epidermis and about 10-15% in the stratum corneum. The expression of aquaporin-3 also decreases with age and high sun exposure and is related to the fact that older skin and very sun-exposed skin are typically drier. A connection has also been seen between the function of aquaporin-3 and a number of skin diseases such as psoriasis.
GLYCERIN
- FUNCTIONS IN THE SKIN
The most important property of glycerin is that it is very effective at binding water - it is hygroscopic. This property gives rise to a lot of different functions in the skin:
- Humectant: Glycerin is effective in binding water to the skin - both water that is in the skin already and water from the surrounding environment binds. Glycerin inhibits the evaporation of water from the surface of the skin.
- Accelerates the recovery of the skin barrier and can protect against irritation: This function is probably related to the fact that glycerin helps to create an environment with moisture that promotes the construction of the barrier. The barrier function is most often measured quantitatively by measuring TEWL, as mentioned above. Various studies have shown that glycerin has a positive effect both as a pre-, during- and post-treatment of skin that is exposed to barrier-degradation. Such a degradation can be to remove some stratum corneum with tape or by exposing the skin to a strong soap substance, which will usually be irritating and increase TEWL. In a study where such skin was post-treated with glycerin, it was shown that glycerin could rehydrate the skin and a longer study showed that glycerin could improve the skin barrier. In another study, the skin was pretreated with either a 10% glycerin emulsion or the same emulsion without glycerin (the vehicle) and then washed with a strong soap. That study showed that glycerin reduced dehydration, irritation, and barrier degradation, as one would otherwise normally see with such a treatment of the skin by washing.
- Inhibits the phase transition of the lipids in the stratum corneum - connection with barrier function: The skin barrier consists mainly of optimal organization and interaction of the stratum corneum components - i.e. the corneocytes and the intercellular lipids, which are mainly fatty acids, ceramides and cholesterol. The extracellular lipids in the stratum can be organized into two phase types: a solid and a more fluid phase organization. The balance between these two phases is determined by the composition of the lipids (and especially the degree of saturation of the fatty acids), the amount of water and probably also other still unknown factors. If the lipids are primarily in the liquid phase, one has only a moderate barrier function, while lipids in the more solid phase give a very poor barrier function and high water loss (TEWL). Dry and cold weather promotes the solid phase and dry skin. The optimal barrier against water loss is obtained by a mix of the two phases. Studies suggest that glycerin may inhibit the transition from liquid to solid phase.
- Accelerate wound healing: Similar to barrier reconstruction, it is likely related to the fact that glycerin promotes a moist environment.
- Keratolytic function: Glycerin has an indirect keratolytic effect - probably again by promoting the moist environment necessary for the keratolytic enzymes. These enzymes break down desmosomes, which are important elements that binds the cells together. This is the above-mentioned desquamation process, which is necessary for the dead skin cells to be shed from the skin. The process is usually well regulated - but in certain skin diseases it does not work optimally.
- Maturation of skin cells: Glycerin has been shown to be involved in the formation of a special lipid (phosphatidylglycerol) which appears to signal certain enzymes involved in skin cell differentiation. Thus, glycerin helps to mature skin cells.
- Smoothing and emollient function: Glycerin can - again through its water-binding property - help to ensure an elastic and well-moisturized skin, with the right mechanical and physiological properties.
GLYCERIN
- PRODUCTIONS AND APPLICATIONS
Glycerin can be produced in different ways and from different starting materials. The most common starting material is vegetable fats, such as corn oil, palm oil and rapeseed oil, but glycerin can also be extracted from animal fats. Via microbial fermentation one can produce glycerin from sugars or carbohydrates and finally glycerin can also be made from petrochemicals - more specifically Propylene (propylene) - but this method is only very rarely used as it is not very advantageous compared to the other methods. In the production via microbial fermentation, yeast cells, certain bacteria or algae are used. This process is a relatively "pure" process but is not widely used industrially.
The production from fats is, as mentioned, the most common and takes place in principle just like in the body: Degradation of triglycerides gives glycerin and three fatty acids. More technically, three different ways are generally used for this degradation - each method has its advantages and disadvantages:
- Hydrolysis, where the ester bonds in the triglycerides are split with high temperature and pressure or via lipase enzymes to obtain glycerin and fatty acids.
- Saponification, where the triglycerides are split by reacting with a base such as Sodium Hydroxide. This releases glycerin and at the same time makes soaps (fatty acid salts)
- Transesterification, where the ester bonds are split using a catalyst (typically a base or acid) and heat and an alcohol (usually methanol or ethanol) is present. This gives glycerin and fatty acid ester, which can be used for e.g. biofuel. There are several sub-methods of transesterification.
The latter method is widely used to produce biofuels and to that end, the glycerin moiety is seen as a residual product (accounting for about 10% of production) to which one must find applications for. It is estimated that the global production of glycerin from biofuel production in 2020 was approx. 42 billion liters.
All methods result in a mix that needs to be purified. First, the glycerin-containing part is separated from the rest. This fraction is rather impure and is called crude glycerin. The impurities that may be present are, for example, inorganic salts, reactants from the production, such as methanol, ethanol and non-decomposed triglycerides, in addition water and partially decomposed triglycerides (di- and mono-glycerides) and soap products. The purification steps depend on the production method and the starting materials used and thus which impurities are present. Some of the most commonly used purification steps are distillation, ion exchange and filtration with activated carbon. You can reach a purity of approx. 99.5%. The glycerin used in cosmetics is usually around 99% pure - and does not normally come from biofuel production, which generally provides a crude glycerin that requires very thorough purification to achieve the standards for glycerin purity needed for cosmetics and medicine application.
Glycerin is used in many different places - for example in food, medicine, cosmetics, in the polymer industry and as a starting material for the manufacture of other substances. A very large portion is used in cosmetics and food.
As a food ingredient, glycerin has the E-number 422 and has been thoroughly assessed with regard to food safety - e.g. by EFSA, which in 2017 assessed that the current permitted use of glycerin in food does not give rise to any safety concerns and that there is no need to restrict the use by an ADI (Accepted Daily Intake). In the food industry (and feed industry) glycerin is used to provide texture, to bind water in the product, which also has an inhibitory effect on microbial growth, to stabilize products and can also be used as a solvent for other food additives. It is not used as a sweetener, although it has a sweet taste.
In medicine, glycerin is used, for example, as a lubricant, moisturizer, and laxative. As mentioned, measurement of glycerin in the liver can be used as a marker for liver disease. In addition, glycerin is used for wound treatment (85% glycerin solution is both antibacterial and antiviral and thus reduces inflammation), in cough medicines and capsules and as an additive in antibiotics.
Glycerin can also be used as a raw material to produce many other substances. For example, to produce lactic acid, 1,3-propanediol, citric acid and butanol. It can be used to make special polymers, such as polyglycerols and polyurethanes. Glycerin is also used as a component in various emulsifiers - and as mentioned for the production of nitroglycerin for explosives and medicines.
Glycerin is also used as an antifreeze - again because it is good at binding water and thereby preventing water from forming crystals. Glycerin is also a good solvent that can be used to make plant extracts, for example.
The cosmetics industry is one of the major buyers of glycerin. Glycerin is used on a very large scale in cosmetics - it is in the top three of the most widely used ingredients in cosmetics and can be used safely in relatively high concentrations. There have been very few reports of side effects - one example is that glycerin can cause short-term irritation when used on damaged skin. The primary purpose of glycerin in cosmetics is to be a humectant and give all the good effects that a well-moisturized skin has. The concentration of glycerin is obviously important for the effect. For example, one study has shown that glycerin above 3% has a moisturizing and skin caring effect, another study shows that 1% also has a positive effect and another study shows that 10% is more effective than 5%. In any case, the effect also depends on the composition of the overall product, but there is no doubt that glycerin can be a very effective and beneficial ingredient in cosmetics.
SOURCES:
Cremer Oleo website; Glycerine. https://www.cremeroleo.de/en/products/glycerine.html. Lokaliseret 28. Februar 2022.
Draelos, Zoe. Aquaporins: An introduction to a key factor in the mechanism of skin hydration. The Journal of clinical and aesthetic dermatology. 2012; 5. 53-56.
EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food), Mortensen, A. et al. Scientific opinion on the re-evaluation of glycerol (E 422) as a food additive. EFSA Journal 2017; 15(3):4720.
Fluhr, J. W.; Gloor, M.; Lehmann, L.; Lazzerini, S.; Distante, F.; & Berardesca, E. Glycerol accelerates recovery of barrier function in vivo. Acta dermato-venereologica. 1999; 79(6), 418–421.
Fluhr, J. W.; Mao-Qiang, M.; Brown, B. E.; Wertz, P. W.; Crumrine, D.; Sundberg, J. P.; Feingold, K. R.; & Elias, P. M. Glycerol regulates stratum corneum hydration in sebaceous gland deficient (asebia) mice. The Journal of investigative dermatology. 2003; 120(5), 728–737.
Fluhr, J.W.; Bornkessel, A.; Berardesca, E. 2006. Glycerol — Just a Moisturizer? Biological and Biophysical Effects. Kapitel 20 In: Loden, M. & Maiback, H. I. Dry Skin and Moisturizers, CRC Press, Boca Raton, FL. 227-244.
Fluhr, J.W.; Darlenski, R.; Surber, C. Glycerol and the skin: holistic approach to its origin and functions. The British journal of dermatology. 2008; 159(1): 23-34.
Gloor, M.; & Gehring, W. Increase in hydration and protective function of horny layer by glycerol and a W/O emulsion: are these effects maintained during long-term use?. Contact dermatitis. 2001; 44(2), 123–125.
Goyal, S.; Hernández, N.B.; & Cochran, E.W. An update on the future prospects of glycerol polymers. Polymer International. 2021; 70: 911-917.
Hara, M.; Ma, T.; & Verkman, A. S. Selectively reduced glycerol in skin of aquaporin-3-deficient mice may account for impaired skin hydration, elasticity, and barrier recovery. The Journal of biological chemistry. 2002; 277(48), 46616–46621.
Hara-Chikuma, M.; & Verkman, A. S. Aquaporin-3 functions as a glycerol transporter in mammalian skin. Biology of the cell. 2005; 97(7), 479–486.
Korponyai, C.; Szél, E.; Behány, Z.; Varga, E.; Mohos, G.; Dura, Á.; Dikstein, S.; Kemény, L.; & Erős, G. Effects of Locally Applied Glycerol and Xylitol on the Hydration, Barrier Function and Morphological Parameters of the Skin. Acta dermato-venereologica. 2017; 97(2), 182–187.
Lodén, M.; Andersson, A. C.; Anderson, C.; Bergbrant, I. M.; Frödin, T.; Ohman, H.; Sandström, M. H.; Särnhult, T.; Voog, E.; Stenberg, B.; Pawlik, E.; Preisler-Häggqvist, A., Svensson, A.; & Lindberg, M. A double-blind study comparing the effect of glycerin and urea on dry, eczematous skin in atopic patients. Acta dermato-venereologica. 2002; 82(1): 45–47.
Medical College Of Georgia. Glycerin May Help Skin Disease, Study Finds. ScienceDaily. 2003, December 3. Lokaliseret 25. Februar, 2022: www.sciencedaily.com/releases/2003/12/031203075525.htm.
PubChem Sketcher V2.4. Lokaliseret 22. Februar 2022: