Vitamin C
Protect skin cell against free radicals
Vitamin C is an antioxidant. Antioxidants protect skin cells against free radicals that cause ageing of the skin. Vitamin C also inhibits melanin production in the skin, which helps reduce hyperpigmentation and brown spots, even out skin tone and improve skin radiance. Vitamin C stimulates the skin’s ability to produce collagen and increases cell renewal. Vitamin C smooths fine lines and improves skin radiance. Vitamin C has a positive effect against inflammation of the skin, inhibits bacteria and can therefore be applied to problem skin. Vitamin C is UV-sensitive and it is recommended to use a SPF +15 sunscreen together with the products.
Our products contain a stable derivative of Vitamin C 3-O-Ethyl Ascorbic Acid.
The effect of using our products can be seen as soon as after the first week, but the best results are seen after 8 weeks of use.
PRODUCTS WITH VITAMIN C
ABOUT VITAMIN C & VITAMIN C- DERIVATIVES
Vitamin C is an important, water soluble vitamin, which, like other vitamins, needs to be supplied through the diet, as the human body cannot produce it. Vitamin C has several very important functions in the body – primarily due to its redox properties, which make it an antioxidant. It is, for example, a co-factor for certain hydroxylating enzymes – some of which are important for the collagen synthesis and others for the synthesis of certain hormones and neurotransmitters – and it is also an important part of and modulator of the immune system.
Depending on age, sex and pregnancy, it is recommended to consume somewhere between 0.2 and 1.0 g of Vitamin C per day. Vitamin C is relatively harmless and will be excreted in the urine at higher doses – at very high doses it can, for example, cause stomach problems. Vitamin C is found throughout the body – and especially in the skin.
Here the focus will be on Vitamin C as well as selected derivatives thereof and the skin.
Vitamin C is found naturally in the body in the form of a redox system which consists of the reduced (and more stable) form L-ascorbic acid and the oxidized (and less stable) form L-dehydroascorbic acid (the D-isomer is also present in the nature but is biologically inactive). Between these two forms, Vitamin C is found as a charged ascorbate and an ascorbyl radical. This redox process is reversible, which means that it can go both ways so that ascorbic acid can be regenerated.
The primary active form is the reduced L-ascorbic acid, where the substance can release a hydrogen atom, whereby it itself becomes ionized to the ascorbate form (an anion) – this process is dependent on pH. At pH 5, most will be in the ascorbate form. Ascorbate can then donate an electron to another molecule so that this other molecule is reduced, while the ascorbate is oxidized to ascorbyl radical, which will then lose another electron and form the oxidized form, dehydroascorbic acid.
This “other molecule” or atom to which ascorbate donates electrons can, for example, be a reactive oxygen species (ROS) that could otherwise damage other molecules in the body’s tissues, such as lipids in the cell membrane or the DNA-molecule. A Vitamin E radical can also be the receiver of an electron from ascorbate, whereby Vitamin E is regenerated to its active antioxidant form. Similarly, Vitamin C can perform its function as a co-factor for certain enzymes, by maintaining metal ion (primarily iron and copper) within these enzymes in a reduced state (delivering an electron to the metal ion), which is required for the enzyme so perform its activity.
The oxidized dehydroascorbic acid can then be reduced back – “recyled” – to ascorbic acid by other enzyme redox systems in the body and the process can run again.
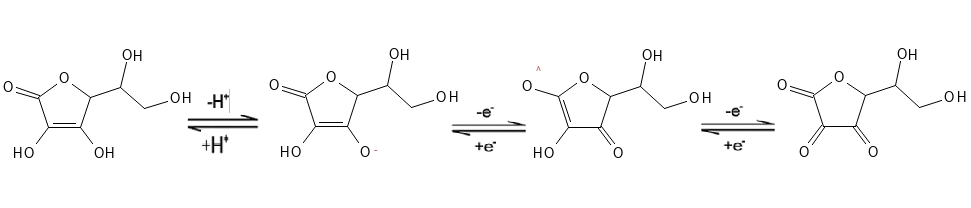
Figure 1. The Ascorbic Acid-Dehydroascorbic Acid redox-process.
Vitamin C’s antioxidant (redox) function is what mainly gives the effects observed with vitamin C – and in relation to the skin there are several very interesting effects, e.g.:
- Vitamin C helps collagen in different ways: by being a co-factor for several hydroxylase enzymes, which plays a role in stabilizing the collagen structure, by promoting the collagen gene expression and by inhibiting the expression and activity of MMP enzymes, which otherwise will degrade collagen.
- Studies also show very clearly that Vitamin C has a positive effect in relation to wound healing. The effect is directly related to its co-factor activity in the synthesis of collagen and its effect on the proliferation and migration of dermal fibroblasts which has an impact on wound healing and the formation of scars.
- Vitamin C’s antioxidant properties also contribute to the inhibitory effect it has against UV-induced damage to the skin. It is believed, among other things, to be active by modulating gene expression and inhibiting the secretion of pro-inflammatory cytokines – and by inactivating reactive oxygen species, which are formed in the skin by UV exposure.
- Vitamin C has also been shown to inhibit melanogenesis (pigmentation in the skin), probably by interacting with and inhibiting tyrosinase, which is the rate-limiting enzyme in melanogenesis, thereby reducing the pigmentation of the skin.
An important point in relation to topical use of Vitamin C is that several studies suggest that a primary factor for whether topical use of Vitamin C has an effect is the person’s plasma Vitamin C level: If the skin is already “saturated” with Vitamin C (which is primarily from oral intake), then an extra topical application will probably not have much effect.
The challenges by using Vitamin C in skin products are related to the fact that ascorbic acid is not very stable. It can be irritating to the skin at too high concentration, and it is very hydrophilic, which means that it does not penetrate the skin very easily. This is tried among other things to overcome by making derivatives of ascorbic acid – which would then be a precursor and should be converted to ascorbic acid in the skin. Several studies show that the vehicle by which Ascorbic Acid and its derivatives are delivered to the skin has an impact on stability – so it is not always easy to compare different stability studies of these substances.
The EU legislation does not set limits on the use of ascorbic acid or the derivatives mentioned here. Below, Vitamin C and the most common Vitamin C derivatives are reviewed.
ASCORBIC ACID
Ascorbic Acid has as mentioned some challenges in relation to use on the skin due to its instability (especially in the presence of heat, oxidative conditions, high pH, UV light and certain metal ions) and its hydrophilic nature which inhibits the penetrate into the skin. Some work is done in making small particles or multi-layered micro-emulsions or very acidic formulations which should be able to alleviate these challenges.
Ascorbic Acid does not have a carboxylic acid group like most organic acids, but an enol group which gives it the acidic properties (and also forms the unstable part of the molecule). It has certain chemical structural similarities with sugar molecules and is naturally produced in the plants and animals (which can synthesize it) from glucose.
Ascorbic Acid is used in cosmetics, but due to its instability can give the product a yellowish color.
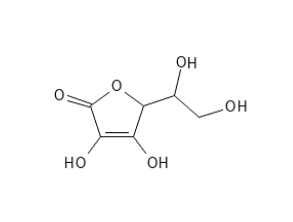
Figure 2. Ascorbic Acid
ASCORBYL PALMITATE
Ascorbyl Palmitate has the basic structure as Ascorbic Acid but has the fatty acid Palmitic Acid added by an ester bond. This makes the molecule more lipophilic – it is an amphiphilic molecule, which helps the penetration into the skin and reduces the potential skin irritation, but there is doubt about how easily it is converted to Ascorbic Acid in the skin.
In terms of stability, Ascorbyl Palmitate is generally more stable than Ascorbic – but not in terms of pH. It degrades gradually under UV light and with moisture.
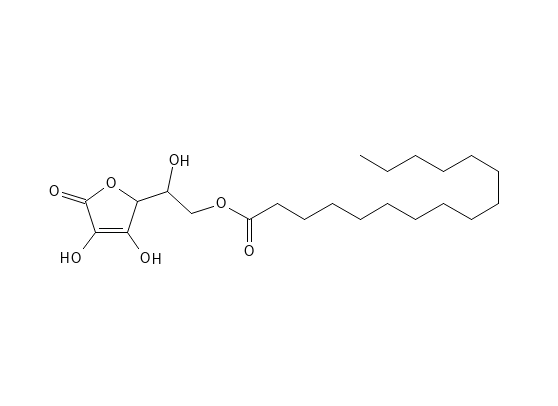
Figure 3. Ascorbyl Palmitate
ASCORBYL GLUCOSIDE
Ascorbyl Glucoside consists of Ascorbic Acid and a glucose molecule that is bonded together by an ether linkage. It is hydrophilic and thus water-soluble, which means that it in itself does not easily penetrate the skin (this can be remedied via the formulation). It has been shown in vivo that Ascorbyl Glucoside can be hydrolyzed to Ascorbic Acid and Glucose.
Ascorbyl Glucoside is much more stable than Ascorbic acid – even in aqueous solutions at high temperature. In relation to the presence of metal ions, this derivative is the most stable – but pH increase can trigger instability – just like the other derivatives. It is quite stable toward UV light.
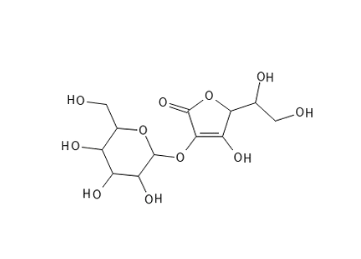
Figure 4. Ascorbyl Glucoside
MAGNESIUM ASCORBYL PHOSPHATE
Magnesium Ascorbyl Phosphate has a phosphate group attached to the ring structure of Ascorbic Acid and Magnesium ion attached. It is hydrophilic and thus water-soluble, which means that it in itself does not easily penetrate into the skin (this can be remedied via the formulation). It can be converted at a relatively slow rate to Ascorbic Acid in the skin via phosphatase enzymes.
Magnesium Ascorbyl Phosphate is generally much more stable than Ascorbic acid and Ascorbyl Palmitate but is not very stable to metal ions and UV light. It is more stable at higher pH values, but unstable at low pH, where e.g. Ascorbic acid is more stable. It is quite stable to UV light.
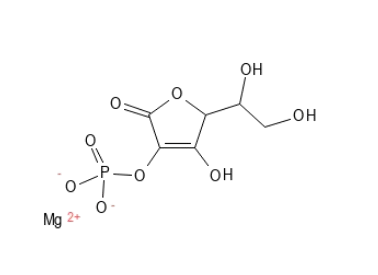
Figure 5. Magnesium Ascorbyl Phosphate
3-0-ETHYL ASORBIC ACID
3-O-Ethyl Ascorbic Acid is quite similar to Ascorbic, the only difference being that one Oxygen atom on the ring structure has become an ether linkage to an ethyl group. This small group makes the molecule more amphiphilic, helping it to get more easily into the skin.
3-O-Ethyl Ascorbic Acid is reasonably stable at different temperatures, the presence of oxygen and UV light. In relation to pH values, this is the most stable among the derivatives reviewed here. Compared to higher temperatures, it is slightly less stable than Ascorbyl Glucoside and somewhat less stable than Magnesium Ascorbyl Phosphate. O-Ethyl Ascorbic Acid and Ascorbyl Glucoside show best stability at room temperature and in general.
When measuring antioxidant capacity, it has been shown in a study that 3-O-Ethyl Ascorbic Acid is approx. half as good as Ascorbic acid and about twice as good as Ascorbyl Glucoside, while Magnesium Ascorbyl Phosphate and Ascorbyl Palmitate had the lowest antioxidative capacity.
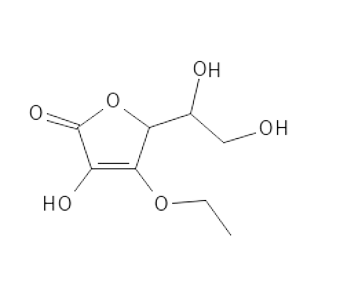
Figur 6. 3-0-Ethyl Asorbic Acid
SOURCES:
Grosso G, Bei R, Mistretta A, Marventano S, Calabrese G, Masuelli L, Giganti MG, Modesti A, Galvano F, Gazzolo D. Effects of Vitamin C on health: a review of evidence. Frontiers in Bioscience. 2013 Jun 1;18:1017-29
Schlueter A. K. and Johnston C. S. Vitamin C: Overview and Update. Journal of Evidence-Based Complementary & Alternative Medicine. 2010 16(1) 49-57
Pullar J. M., Carr A.C., Vissers M. C. M. The Roles of Vitamin C in Skin Health. Nutrients. 2017 Aug 12;9(8):866
Al-Niaimi F., Chiang N. Y. Z. Topical Vitamin C and the Skin: Mechanisms of Action and Clinical Applications. Journal of Clinical Aesthetic Dermatology. 2017 Jul;10(7):14-17.
Silva, S. R. Comparative study of ascorbic acid and derivatives with interest in anti-aging cosmetics. Master in Pharmaceutical technology. Porto, September 2017. Lokaliseret den 22. Juni 2021 på: https://repositorio-aberto.up.pt/bitstream/10216/108653/2/228901.pdf