Collagen
Collagen
Collagen is a protein made naturally within the body and is the foundation of our skin, hair, and bones.
It’s responsible for making our skin healthy, youthful, and plump. Because it’s created naturally by our bodies, collagen production begins to slow down as we age. Other factors such as sun exposure, smoking, or sugar consumption also contribute to collagen loss. This loss in collagen will result in a loss of fullness, dullness, and fine lines and wrinkles.
Our collagen serum stimulate collagen production and create a more smooth and plump appearance in your skin.
Collagen serum can be used in combination with all our other serums.
We use Collagen BioStine HP (f) from the company Bio-Nest, Taiwan. BioStine HP (f) Collagen originates from fish scales, which primarily contain type I Collagen. This raw material consists of Hydrolyzed Collagen, as well as Euglena Gracilis Polysaccharide, which are complex carbohydrates from the Algae Euglena Gracilis.
PRODUCTS WITH COLLAGEN
EVERYTHING YOU NEED TO KNOW
ABOUT COLLAGENCollagen is a group of 28 different types of proteins and is the most common protein in the animal kingdom. Evolutionary, collagen is a very “old” protein – for example, intact collagen has been found in 68-million-year-old Tyrannosaurus rex fossils. In the human body, collagen makes up about 30% of the entire protein mass and about 75% of the dry weight of the skin is collagen. Collagen is found especially in connective tissue – for example in bones, cartilage, tendons, joints and the skin.
The 28 types of collagens are designated by Roman numerals the number according to when they were discovered – there is e.g. Collagen I (the prototype that was first discovered), Collagen II, Collagen III, etc. They can be divided into 8 families, of which e.g. Collagen I and III belong to the fibril-forming collagens, while e.g. Collagen XIII belongs to the transmembrane collagens. Common to all collagens – what defines a collagen molecule – is a special structure in the molecule called a triplex helix, which consists of three chains of amino acid (peptides) that twist right-handed around each other and form a very stable structure. What primarily makes the 28 types of collagens different are the other segments (domains) that are in the protein structures and the three-dimensional structures they form. The triplex helix structure thus constitutes a larger or smaller part of all the collagen types – for example, Collagen I consists of 96% triplex helix structure, while it is less than 10% in Collagen XII.
PROPERTIES OF COLLAGEN IN THE SKIN
In the skin, 70-90% of the collagen is Collagen I, while 10-20% is Collagen III – there are smaller amounts of e.g. Collagen V, VII and XVII. They are found especially in the extracellular matrix (between the cells) together with other substances such as elastin and glycosaminoglycans (e.g. hyaluronates and dermatan sulphate), where it i.a. gives structure and elasticity to the skin. Collagen is also important in the wound healing process. It is seen, for example, in the disease scurvy, where symptoms such as bleeding gums and incomplete wound healing occur. This is because a lack of Vitamin C means that no proper collagen is produced, as vitamin C is an important co-factor for certain enzymes, which play a crucial role in the formation of the triplex helix structure in collagen. There are also a large number of other diseases that are due to defects in the formation of collagen or direct mutations in the collagen genes.
WHAT HAPPENS TO COLLAGEN WHEN THE SKIN AGES?
There is a certain turnover of collagen, which is quite precisely controlled and varies with age. Collagen and elastin are fairly stable proteins, whose half-life is measured in year – each molecule is thus exposed to a lot along the way and thus continuously accumulates minor damage in its structure, which impairs its function. Matrix Metalloproteinases (MMPs) are the enzymes that start the breakdown of collagen – e.g. Collagen I, II and III are cleaved by i.a. MMP-1, MMP-8 and MMP-13 – after which other degrading enzymes continue to break down the collagen components. The activity of MMP enzymes is associated with UV radiation and oxidative stress in the skin caused by free radicals. As one gets older, the production and quality of collagen in the skin decreases. The content of collagen peaks around the age of 30 and thereafter the amount is reduced by about 1-1.5% per year – together with associated extracellular components, so that when you are around 70 years, the amount is about 25% of max. The decrease in collagen content and the quality of the collagen is correlated with aging of the skin such as wrinkles.
COLLAGEN BIOSYNTHESIS
Proteins are synthesized according to the same basic procedure: The central dogma, which describes how proteins are synthesized based on the genetic information contained in the DNA. In short, the gene in the DNA (after a signal has arrived) is “opened” so that it can be copied (transcribed) into an RNA molecule called a messenger RNA (mRNA). This happens inside the cell nucleus. The mRNA molecule enters the cytoplasm of the cell, where special units called ribosomes translate (translate) the mRNA code into amino acids that are put together into a peptide chain – a long chain of amino acids. This is slightly modified in some (post-translational modification), after which it is folded into the very specific three-dimensional structure – possibly together with other peptide chains to form the final protein. Thus, proteins are generally very large molecules, which may consist of several peptide chains.
In the following description of the synthesis of collagen and its structure, the focus will be on Collagen I, which is the most abundant collagen in the skin.
Fibroblasts are skin cells in the dermis that produce most of the Collagen I in the skin. On the surface of the cell there are a large number of receptors, some of which can bind signaling molecules that lead to the activation of the cell and start the process of producing collagen. Inside the cell nucleus, the specific genes for the three peptide chains are transcribed into mRNA, which is then released into the cell’s cytoplasm, where they are translated via ribosomes into amino acids that are assembled into peptide chains.
Each peptide chain consists of 1050 amino acids and for Collagen I it consists of two α1 (I) chains and one α2 (I) chain (other collagens consist of three identical chains or three different chains). What is special about collagen is that the amino acid frequency that is generally repeated throughout the chain is Glycine-X-Y, where X and Y are usually the amino acids proline and hydroxyproline – it is this amino acid sequence that is crucial for the formation of the important triplex helix structure. The peptide chains are led directly into a membrane system organelle called the endoplasmic reticulum, where the three procollagen peptide chains undergo a number of processes (post-translational modifications) – i.a. glycosylation, where certain sugar molecules are bound in certain positions of the chain, hydroxylation of certain proline and lysine amino acids, and finally disulfide bonds between the three chains are made, which cause them to lock together in the well-known right-handed triplet helix – a bit like a zipper. This procollagen is then by vesicles led out of the cell to the extracellular space, where extracellular enzymes remove the ends of the procollagen. This results in the structure called tropocollagen, which for Collagen I basically consists only of a triplet helix, which is 300 nm long and about 1.5 nm in diameter. This removal of the ends allows the molecule to form crosslinks to other triplex helices of collagen I and thus form larger structures called collagen fibrils, which have a special structure that in electron microscopy appears as a striped structure. Collagen in fibrils has a diameter of 50-200 nm. The fibrils can then assemble into even larger fiber structures of collagen I, which, for example, in tendons are up to 1 cm long and 500 nm in diameter.
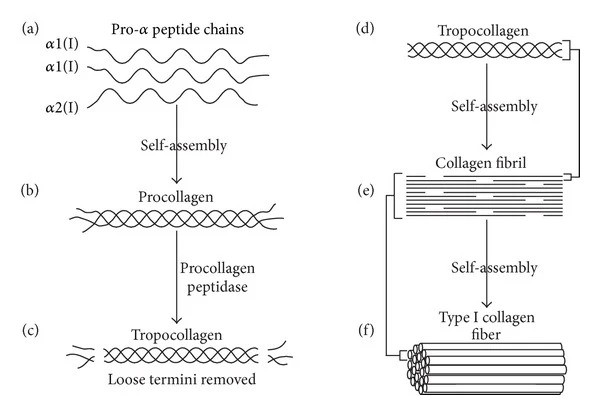
Figure 1 Collagen I biosynthesis: (a) Two identical α1(I) and one α2(I) peptide chains self-assemble to form procollagen (b). (c) The enzyme procollagen peptidase removes “loose” ends to create tropocollagen (d). Tropocollagen molecules self-assemble to from a growing collagen I fibril (e). These collagen fibrils can afterwards selv-assemble to form Collagen I fiber (f). This figure is from the article ”Collagen Scaffolds in Bone Sialoprotein-Mediated Bone Regeneration” by T. E. Kruger, A. H. Miller and J. Wang. Published by Hindawi in The Scientific World Journal, vol. 2013, 6 pages, 2013.
COLLAGEN IN COSMETICS
Collagen is a very large and insoluble structure which will not be able to get through the skin; – but it can form a film (cohesive structure), which reduces water loss from the skin and thus has a moisturizing effect – also because collagen can bind water to itself.
In many cases, cosmetics use hydrolyzed collagen, which has typically first been denatured and then enzymatically broken down into smaller parts, which can vary greatly in size and capacity to penetrate the skin. For example, it has been shown that some collagen hydrolysates have antioxidant and moisturizing properties and repairing properties for damaged skin.
There are several natural sources of collagen: pigs, cattle and marine animals such as fish. Bovine collagen is readily available and useful for some biomechanical purposes, but unfortunately quite heterogeneous. It can also be immunogenic (can cause an immune reaction) and lose its structural integrity during the isolation process and together with collagen from pigs, there is a risk of zoonotic diseases, such as BSE (Bovine Spongiform Encephalopathy – mad cow disease). Collagen from a marine source is less heat stable but has properties that make it interesting in a cosmetic context. As with other natural sources, there is a risk of batch-to-batch variations that can be difficult to control.
Another way is to synthesize smaller peptides in the laboratory with the same or similar amino acid sequence as in collagen – it can be small di- or tripeptides (consisting of two or three amino acids) or larger structures that can form triplex helix similar to those in collagen. Another way is to produce genetically modified cells (e.g. cells from barley or the tobacco plant) which can produce collagen under more controlled conditions.
THE USE OF COLLAGEN
In addition to the use in cosmetics, collagen is used in many other contexts – e.g. as fillers, where a small amount of collagen is injected into the skin to reduce e.g. wrinkles and in medical contexts for e.g. wound healing. Collagen is also available as a dietary supplement (typically along with a variety of vitamins and other natural substances), the purpose of which is to improve the structure of the skin. Some studies show effects on the skin with very complex supplements where collagen is included. The theory behind this is that the collagen after ingestion is broken down in the stomach into primarily amino acids and about 10% becomes small di and tri-peptides, which are absorbed into the blood and distributed around the body – e.g. to the skin, where the cells can use the amino acids as “building blocks” for biosynthesis of collagen. Studies show that di-peptides with hydroxyproline can stimulate fibroblasts via receptors and thus induce collagen synthesis.
SOURCES:
Aguirre-Cruz, G.; León-López, A.; Cruz-Gómez ,V.; Jiménez-Alvarado, R., Aguirre-Álvarez, G. Collagen Hydrolysates for Skin Protection: Oral Administration and Topical Formulation. Antioxidants (Basel). 2020 Feb 22;9(2):181.
Avila Rodríguez, M. I.; Rodríguez Barroso, L.G.; Sanchez, M. L. Collagen: A review on its sources and potential cosmetic applications. Journal of Cosmetic Dermatology 2018; 17: 20-26.
Kruger, T. E.; Miller, A. H.; Wang, J. Collagen Scaffolds in Bone Sialoprotein-Mediated Bone Regeneration. The Scientific World Journal, vol. 2013, Article ID 812718, 6 pages, 2013
Lodish, H., Berk, A.; Zipursky, S.L., et al. Molecular Cell Biology. 4th edition. New York: W. H. Freeman; 2000. Section 22.3, Collagen: The Fibrous Proteins of the Matrix.
Lokaliseret 19. Juli 2021: https://www.ncbi.nlm.nih.gov/books/NBK21582
Reilly, D.M.; Lozano, J. Skin collagen through the lifestages: importance for skin health and beauty. Plastic and Aesthetic Research 2021; 8:2.
Ricard-Blum, S. The collagen family. Cold Spring Harbor perspectives in biology. 2011 Jan 1;3(1):a004978.
Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annual review of biochemistry, 2009, vol. 78, 929–958.
Sionkowska, A.; Adamiak, K.; Musiał, K.; Gadomska, M. Collagen Based Materials in Cosmetic Applications: A Review. Materials 2020, 13, 4217.




